Cross-Contamination: How Medications Get Mixed Up and What It Means for Your Safety
When a drug meant for one patient accidentally ends up in another’s vial, that’s cross-contamination, the unintentional mixing of pharmaceutical substances during manufacturing, handling, or dispensing. Also known as drug contamination, it’s not just a lab error—it’s a patient safety crisis. This isn’t science fiction. In 2012, a fungal outbreak from contaminated steroid injections killed 64 people and sickened over 750 across 20 states. The source? A compounding pharmacy that didn’t separate sterile and non-sterile areas. That’s cross-contamination in action—quiet, deadly, and preventable.
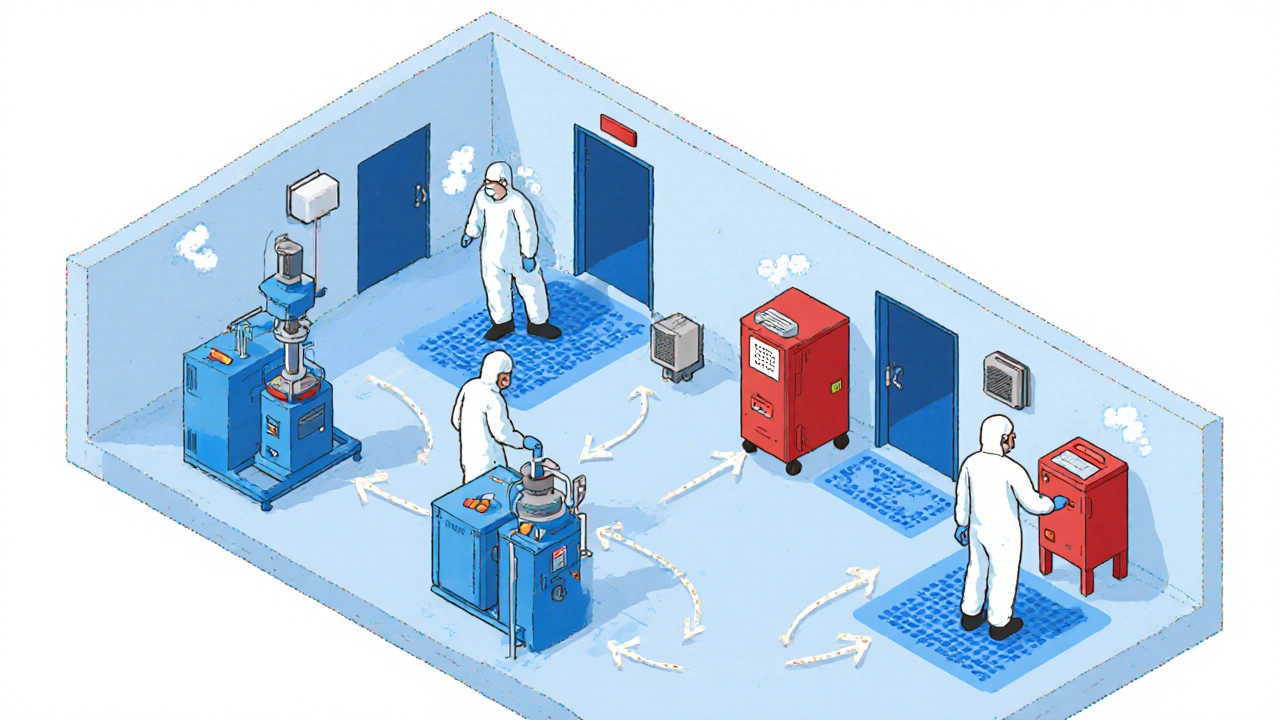
Sterile compounding, the process of preparing injectable drugs in controlled environments is where most serious contamination risks occur. Hospitals, pharmacies, and compounding centers handle hundreds of drugs daily. If a technician uses the same needle, glove, or surface for multiple medications—say, switching from chemotherapy to an IV antibiotic without proper cleaning—traces of one drug can pollute the next. Even tiny amounts matter. A single particle of a potent drug like fentanyl or doxorubicin can trigger a life-threatening reaction in someone not prescribed it. And it’s not just injectables. Oral meds, topical creams, and even inhalers can be contaminated if storage or packaging protocols fail.
Pharmaceutical hygiene, the strict practices used to keep drugs free from foreign substances is the shield against this. It includes using dedicated equipment, changing gloves between tasks, cleaning surfaces with validated disinfectants, and testing final products for microbes or chemical residues. But it only works if every step is followed. Many smaller pharmacies cut corners to save time or money. And when that happens, patients pay the price. You might not see the contamination—but your body will. Allergic reactions, organ damage, or unexpected drug interactions can follow.
What’s surprising is how often this happens out of sight. You won’t find cross-contamination listed on your prescription label. It’s hidden in the supply chain—in bulk drug shipments, in shared IV bags, in multi-dose vials reused beyond safety limits. That’s why hospital pharmacies are on the front line. They’re the ones catching shortages, tracking recalls, and spotting inconsistencies in drug batches. The posts below show how this issue connects to real-world problems: sterile injectable shortages, drug interactions, and even antibiotic resistance when contaminated meds trigger unintended immune responses.
Below, you’ll find real stories from the field—cases where cross-contamination nearly killed someone, where testing caught it in time, and where protocols failed because no one asked the right questions. These aren’t theoretical risks. They’re documented events. And the fixes? They’re simple, but only if you know what to look for.
Contamination controls in generic drug manufacturing prevent dangerous adulteration through strict cleaning protocols, cleanroom design, and real-time monitoring. Learn how facilities stay compliant and avoid costly recalls.