Contamination Controls: Preventing Adulteration in Generic Pharmaceutical Manufacturing
Why contamination control isn't optional in generic drug manufacturing
Generic drugs make up 90% of all prescriptions filled in the U.S., but they account for only 22% of total spending. That means manufacturers are under constant pressure to cut costs-without cutting corners. One wrong move, one missed swab, one uncleaned hopper, and an entire batch can become adulterated. And when that happens, it’s not just a financial loss. It’s a public health risk.
The FDA issued 1,042 warning letters to pharmaceutical companies in 2022. Nearly 37% of them cited contamination issues. That’s not a glitch. It’s a pattern. And it’s happening most often in generic drug plants. Why? Because many of these facilities operate on razor-thin margins. They can’t afford the $185 million cleanrooms that innovator companies build. So they rely on processes-tight, repeatable, documented processes-to keep things safe.
But processes fail when people get tired. When shifts change. When cleaning validation takes five days to get results. When someone skips a gowning step because they’re running late. That’s why contamination control isn’t about fancy equipment. It’s about design, discipline, and detection.
What counts as adulteration-and why it matters
Under 21 CFR 210.3(b)(3), a drug is adulterated if it’s made, packed, or stored under conditions that could make it unsafe. That includes anything from dust particles to leftover active ingredients from a previous batch. Even a few nanograms of a potent drug like a steroid or chemotherapy agent left on a surface can cause serious harm if it ends up in a different product.
The 2020 Valsartan recall is a textbook example. Nitrosamine contaminants showed up in blood pressure meds made by 22 different generic manufacturers. The contamination didn’t come from the raw materials. It came from a solvent used in the synthesis process. One change, one untested step, and suddenly millions of pills were unsafe. The industry lost $1.2 billion in recalled product. That’s not just a cost-it’s a loss of trust.
Regulators don’t wait for disasters to act. The FDA’s 2023 draft guidance says all generic drug manufacturers must set health-based exposure limits (HBELs) for every product by 2025. That means knowing exactly how little of a substance can harm a patient. And then proving your cleaning process removes it to levels below that threshold. No guessing. No assumptions. Just data.
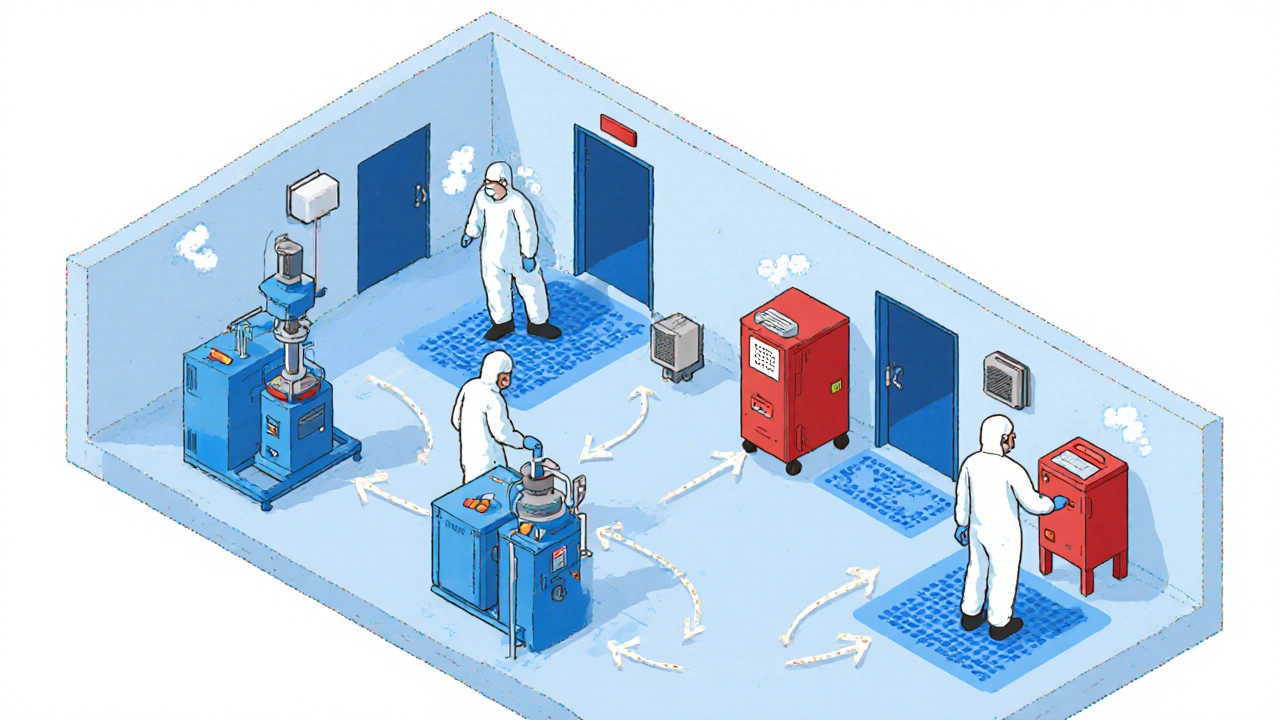
How cleanrooms and airflow keep drugs safe
Generic manufacturers don’t need the same cleanrooms as biologics makers. But they still need structure. Most solid oral dosage facilities use ISO Class 8 (Grade D) for general production, ISO Class 7 (Grade C) for weighing and blending, and ISO Class 5 (Grade A) for any sterile filling-though that’s rare in generics.
Airflow is everything. HVAC systems must push 20 to 60 air changes per hour, depending on the zone. Pressure differentials between rooms must stay between 10 and 15 Pascals. That’s not a suggestion. It’s a requirement under EU GMP Annex 1 (2022). If air flows the wrong way-say, from a dusty packaging area into the blending room-you’ve already lost control.
Modern plants use unidirectional flow: materials and people move in one direction, never backtracking. Airlocks separate zones. Color-coded equipment prevents mix-ups. One facility in Ohio reduced contamination events by 65% just by painting all tablets presses blue and all capsules machines red. Simple. Effective. No training needed.

Cleaning validation: the real test of control
Everyone says they clean their equipment. But how do you prove it?
Traditional swabbing and rinse sampling take 5 to 7 days to get results. By then, the batch is already made. If the test fails, you scrap it. That’s expensive. And risky.
Today’s best practices use rapid microbiological methods (RMMs). These systems detect microbial growth in 24 to 48 hours. Some even use ATP bioluminescence-flashlight-like devices that light up when they touch organic residue. They give you a number in five minutes. Correlation to traditional culture? 95%. That’s not a guess. It’s science.
Chemical residue limits? Most facilities aim for under 10 parts per million (ppm) of any previous drug. For high-potency compounds, it’s 1 nanogram per square centimeter. That’s like finding a single grain of salt in a swimming pool. To hit that, you need validated cleaning procedures, trained staff, and regular revalidation.
One generic manufacturer in India cut their cleaning validation time by 60% by switching to ValGenesis software. But here’s the catch: it took 147 hours of training per operator. That’s three full weeks. If your team isn’t trained, the system fails.
Human error is the biggest source of contamination
Dr. Michael Gamlen, a leading pharma consultant, says 83% of contamination events come from people. Not machines. Not air. People.
Think about it. You’ve got operators in gowns, gloves, masks. They move between zones. They touch surfaces. They talk. They sneeze. They forget to change gloves. They take a shortcut because they’re tired. A 2021 AstraZeneca study found gowning compliance dropped 40% after an 8-hour shift. That’s not negligence. It’s fatigue.
One Teva facility in New Jersey saw a 30% spike in particle counts after switching to reusable isolation gowns. Why? The gowns were harder to clean properly. They had to invest $185,000 in new air showers just to bring counts back down.
Simple fixes work. Dycem CleanZone mats-those sticky blue mats at doorways-reduce foot-borne contamination by 72%. One Pfizer generics site reported that. Staggered shift changes reduce traffic in clean areas. Color-coded tools prevent cross-use. One batch at a time production means no residue from the last product lingers.

Technology is changing the game-but it’s not cheap
Real-time particle counters like the MetOne 3400+ cost $15,000 to $25,000 each. But they cut contamination incidents by 63%. Manual checks miss 78% of transient events. That’s not a luxury. That’s a safety net.
AI-powered systems like Honeywell’s Forge Pharma are now predicting contamination risks before they happen. In a Merck pilot, false alarms dropped by 68%. That means less downtime, fewer batch rejections.
But here’s the reality: not every generic manufacturer can afford this. A full contamination monitoring system can cost $500,000 to $2 million. That’s why 89% of the top 50 generic makers use these systems-but only 37% of small facilities do.
Dr. Paul Garmory warns that over-engineering containment for low-risk products can cost $2.8 million a year with no added safety. The key is risk-proportionate control. Don’t put a Class 5 cleanroom around a tablet press making aspirin. But do put it around a press making a cancer drug.
What’s coming next-and what you need to do now
By 2025, every generic drug maker must have HBELs for every product. That means you need to know the toxicity of every ingredient, every impurity, every cleaning solvent. You need to prove your cleaning removes it to safe levels. The Generic Pharmaceutical Association estimates this will cost $1.2 million per facility.
At the same time, ICH Q13 guidelines for continuous manufacturing are rolling out. These integrate contamination controls directly into the production flow. No more batch testing. No more waiting. Real-time monitoring becomes the standard.
And sustainability is pushing change too. Waterless cleaning systems are now being used by companies like GSK. They cut utility costs by 22% and reduce waste. That’s not just green. It’s smart.
If you’re a small manufacturer, the pressure is real. But the tools are getting more accessible. Cloud-based validation software. Portable ATP testers. Open-source risk assessment templates from ISPE. You don’t need a Fortune 500 budget. You need a plan.
Start with your biggest risk. Map your process. Identify where contamination could happen. Test your cleaning. Train your people. Document everything. Don’t wait for an FDA warning letter. Because by then, it’s too late.
How to start improving your contamination controls today
- Do a risk assessment using ICH Q9. List every step where contamination could occur. Prioritize by risk level.
- Switch to rapid microbiological methods if you’re still using 5-day cultures. Even a basic ATP tester can cut validation time in half.
- Use color coding for equipment, tools, and zones. No training required. No confusion.
- Install sticky mats at all cleanroom entrances. They’re cheap. They work.
- Train staff on gowning every quarter. Fatigue is your enemy. Rotate shifts to keep compliance high.
- Start building HBELs now-even if the deadline is 2025. It’s a year-long project.
Contamination control isn’t about perfection. It’s about consistency. It’s about knowing what you’re doing, proving it, and never stopping.





Comments (11)
Katie Magnus
19 Nov 2025
So let me get this straight... we're risking people's lives because some company didn't want to spend a few million on a cleanroom? 😭 I mean, come on. This isn't a budget spreadsheet, it's medicine. People are dying from this stuff and we're talking about color-coded tools like it's a kindergarten art project.
King Over
19 Nov 2025
Sticky mats work
Johannah Lavin
20 Nov 2025
OMG I just read this and I'm crying 😭 I didn't realize how much goes into making a simple pill safe. Like... those sticky blue mats? I thought they were just for fancy offices but they actually save lives?? 🙌 And the part about fatigue making people skip gowning?? That hits so hard. We need to treat these workers like heroes, not replaceable cogs. 💙
Ravinder Singh
22 Nov 2025
In India, we face this daily. The pressure to cut costs is insane. But I've seen plants where operators train for months on ATP testers - and it works. One plant in Hyderabad cut contamination by 70% using just $2k handheld devices and daily huddles. No fancy AI needed. Just discipline. And yes, training takes time - 147 hours? That's nothing compared to a recall. 💪
Russ Bergeman
24 Nov 2025
Wait. Wait. Wait. You said 'color-coded equipment'? So... you're telling me that painting machines different colors is a legitimate contamination control strategy? That's not science. That's a preschool activity. And who approved this? Did someone get a bonus for this? Also, 'Dycem mats'? That's a brand name. You're recommending a product. That's not neutral. That's advertising. And why is everyone ignoring the real issue? The FDA is broken.
Dana Oralkhan
24 Nov 2025
I work in a small generic lab and this article felt like it was written for us. The part about fatigue? Yeah. My team works 12-hour shifts. They’re exhausted. We started rotating gowning checks every hour instead of once a day - and compliance jumped. No fancy tech. Just paying attention. Also, thank you for mentioning HBELs. We’re starting ours next month. It’s scary, but we’re doing it. 💪
Jeremy Samuel
24 Nov 2025
cleanrooms are overrated tbh. i mean, air flow? pfft. my cousin works at a pharma place in oz and they just wipe stuff down with vinegar and call it a day. 90% of the time its fine. why pay 2 mill for a fancy machine when a rag and some elbow grease works? plus, who even checks if the airflow is 15 pascals? no one. its all theatre.
Destiny Annamaria
26 Nov 2025
I’m from Puerto Rico and we get a lot of these generics here. I used to think 'generic' meant 'cheap'... now I realize it means 'made by people who are barely holding it together.' I’m so grateful for these workers. And I’m so mad that we don’t give them more support. If you’re reading this - thank you. Seriously. You’re doing god’s work. 🙏❤️
Ron and Gill Day
27 Nov 2025
This is a joke. 10 ppm? 1 nanogram? You’re telling me we’re spending millions to detect a grain of salt in a pool? That’s not science - that’s paranoia. The FDA is running a fear campaign. Real contamination? It’s almost nonexistent. This whole thing is a money grab. Stop scaring people with nanograms. People die from aspirin overdoses. Not from 'residue.'
Alyssa Torres
28 Nov 2025
I just want to say - this isn’t just about compliance. It’s about dignity. When you treat your workers like they’re part of a machine, they become part of a machine. But when you train them, listen to them, give them breaks, and say 'thank you' - they become guardians. I’ve seen it. One facility in Ohio started letting operators name their machines. One guy named his tablet press 'Betsy.' He never skipped cleaning it again. Because Betsy was family. ❤️
Dana Dolan
30 Nov 2025
i read this and thought of my uncle who works in a generic plant in dublin. he said they use the same swabs as the hospital but reuse them because 'theyre still clean'. i told him that’s not how it works. he laughed. then he got a warning letter last month. now he’s doing the training. he’s 58. he hates tech. but he’s learning. that’s the real story. not the machines. the people.