Brain Tumors: Types, Grades, and Multimodal Treatments Explained
When you hear the word brain tumor, it’s easy to picture the worst-case scenario. But not all brain tumors are the same. Some grow slowly over years. Others spread aggressively within weeks. What separates them isn’t just location or size-it’s type, grade, and molecular profile. Understanding these factors changes everything: how doctors treat you, what your prognosis looks like, and even how you plan your life after diagnosis.
Not All Brain Tumors Are Created Equal
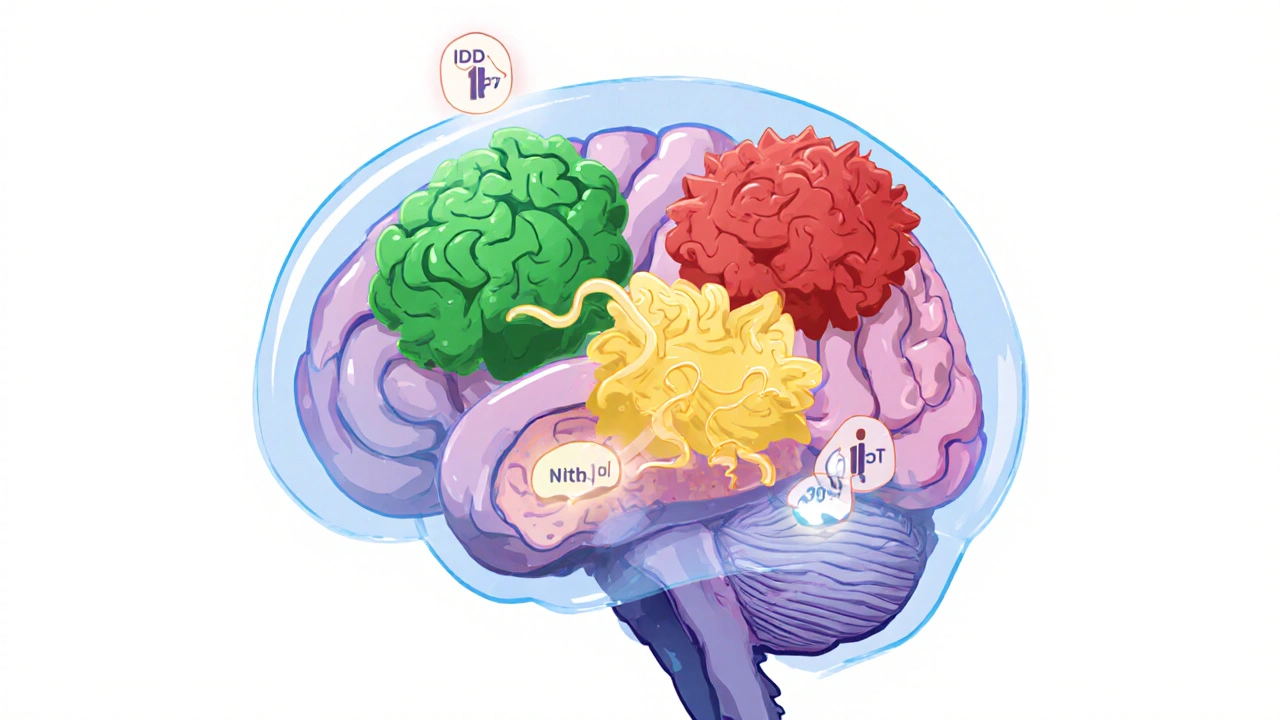
There are over 120 different types of brain tumors. Some start in brain cells. Others begin in the membranes around the brain, nerves, or pituitary gland. The most common primary brain tumors in adults are gliomas-tumors that come from glial cells, which support and protect neurons. Among these, astrocytomas and oligodendrogliomas make up the majority.
Then there are meningiomas, which grow from the tissue covering the brain and spinal cord. These are usually benign but can still cause serious problems if they press on critical areas. Pituitary adenomas, schwannomas, and ependymomas are other frequent types. Each behaves differently. A meningioma might stay put for decades, while a glioblastoma can double in size in under two weeks.
What matters most isn’t just where it starts, but what’s happening inside the cells. That’s where molecular testing comes in.
The WHO CNS5 Grading System: A Revolution in Diagnosis
In 2021, the World Health Organization released its fifth edition of the Classification of Tumors of the Central Nervous System (WHO CNS5). This wasn’t just an update-it was a rewrite. For the first time, diagnosis required more than just looking at cells under a microscope. Molecular markers became mandatory.
Before 2021, a tumor might be called an anaplastic astrocytoma and automatically labeled Grade III. Now, doctors must answer: Is it IDH-mutant? Is 1p/19q codeleted? Is the MGMT promoter methylated? These answers change the grade, the treatment, and the survival outlook.
Here’s how the new system works:
- Grade I: Slow-growing, often curable with surgery alone. Pilocytic astrocytoma is the classic example. It looks almost normal under the microscope.
- Grade II: Low-grade but infiltrative. Cells look slightly abnormal and can spread into healthy brain tissue. These can turn into higher-grade tumors over time. IDH-mutant astrocytomas and 1p/19q-codeleted oligodendrogliomas fall here.
- Grade III: Anaplastic. Cells multiply fast and invade surrounding tissue. These are malignant. Anaplastic astrocytoma (IDH-mutant) and anaplastic oligodendroglioma (IDH-mutant and 1p/19q-codeleted) are examples.
- Grade IV: The most aggressive. Glioblastoma, IDH-wildtype, is the most common and deadly. It forms new blood vessels, kills its own center (necrosis), and spreads rapidly. But here’s the twist: IDH-mutant glioblastoma is now classified separately. It behaves more like a slow-growing tumor with a much better survival rate.
Meningiomas are graded on their own scale: Grade I (benign), Grade II (atypical), and Grade III (anaplastic). They don’t use the same molecular markers as gliomas.
Why Molecular Markers Change Everything
Two tumors that look identical under the microscope can have completely different outcomes based on their genetics.
Take IDH mutation. If a glioma has a mutation in the IDH1 or IDH2 gene, it’s biologically distinct from one without it. IDH-mutant tumors grow slower, respond better to treatment, and live longer. A patient with an IDH-mutant Grade IV astrocytoma has a median survival of 31 months. Without the mutation? Just 14.6 months.
Then there’s 1p/19q codeletion. If both chromosome arms are lost together, the tumor is almost certainly an oligodendroglioma. These tumors respond dramatically well to chemotherapy-especially PCV (procarbazine, lomustine, vincristine). Patients with codeleted Grade II or III oligodendrogliomas can live over 10 years with proper treatment.
And MGMT promoter methylation? That’s a predictor of how well a tumor will respond to temozolomide, the standard chemo drug for glioblastoma. Methylated tumors are more likely to shrink. Unmethylated? The drug may not help much.
These aren’t just academic details. They’re the difference between getting the right treatment-or the wrong one.
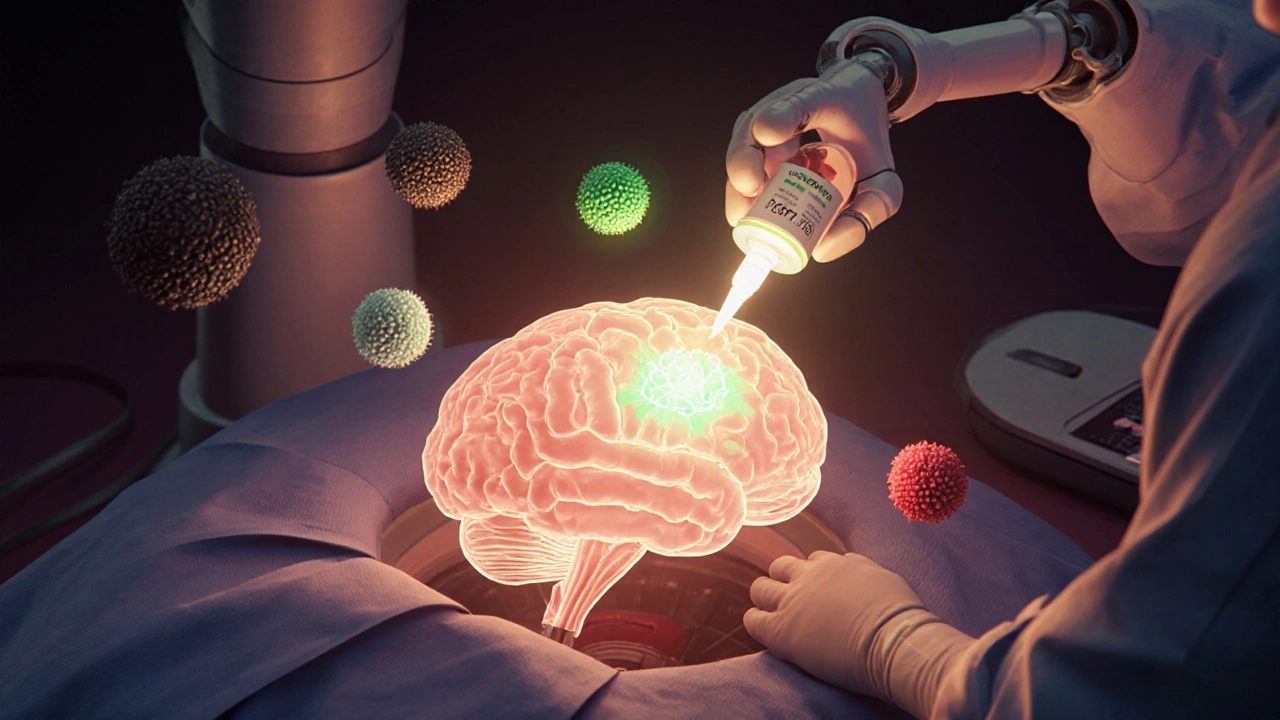
How Treatment Has Changed in the Last Five Years
Traditional brain tumor treatment followed a simple pattern: surgery, radiation, chemo. That’s still the backbone. But now, treatment is layered-multimodal-and personalized.
Surgery: The goal is always maximal safe resection. Surgeons use real-time mapping to avoid damaging speech, movement, or vision centers. In some cases, fluorescent dye (5-ALA) helps highlight tumor tissue during surgery, improving removal accuracy.
Radiation: Focused beams target tumor cells while sparing healthy brain. Techniques like IMRT and proton therapy are now standard. For low-grade tumors, radiation may be delayed until growth is seen. For high-grade tumors, it starts right after surgery.
Chemotherapy: Temozolomide remains the go-to for glioblastoma. But for IDH-mutant Grade II gliomas, the game changed in June 2023. The FDA approved vorasidenib, the first oral drug specifically designed to block the IDH mutation. In the INDIGO trial, it cut the risk of tumor progression by 61% compared to placebo. Patients lived nearly 28 months without their tumor growing-more than double the time with placebo.
Targeted therapy and immunotherapy: These are still experimental for most brain tumors, but trials are ongoing. CAR-T cells, checkpoint inhibitors, and vaccines are being tested, especially for recurrent glioblastoma.
For some patients, watchful waiting is the best option. A small, asymptomatic Grade II tumor might be monitored with MRI scans every 3-6 months. Treatment only starts when signs of growth appear.
What Patients Are Really Facing
Behind the statistics are real people making impossible choices.
A 32-year-old woman diagnosed with a Grade II oligodendroglioma was told she had 72 hours to freeze her eggs before surgery. That’s not unusual. Many treatments affect fertility. Cognitive changes-memory loss, brain fog-are common after radiation. Some patients lose the ability to drive. Others struggle with depression or anxiety long after treatment ends.
Diagnostic delays are still a problem. A 2022 survey found 68% of patients waited more than eight weeks for a confirmed diagnosis. For low-grade tumors, the wait averaged 14 weeks. That’s not just frustrating-it’s dangerous. A slow-growing tumor can become aggressive while you wait.
And misinformation is widespread. A 2022 study found 42% of patients thought a Grade 2 tumor meant a 20% survival chance. It doesn’t. Grade 2 means low-grade. Most people live for many years. The real danger is underestimating the risk of progression.
The Future Is in Liquid Biopsies and Real-Time Monitoring
Right now, confirming a tumor’s type or recurrence requires a biopsy or an MRI. But that’s changing.
Researchers are developing liquid biopsies-blood or spinal fluid tests that detect tumor DNA. A 2023 study in Nature Medicine showed these tests could detect brain tumor DNA in cerebrospinal fluid with 89% accuracy. That means doctors could monitor treatment response without repeated surgeries or scans.
The CODEL trial, which is testing whether combining chemotherapy and radiation gives better results for oligodendroglioma, is expected to release results in late 2024. If successful, it could become the new standard.
And the cost? Molecular testing adds $3,200 to $5,800 to the diagnostic bill. But the payoff? Better outcomes, fewer unnecessary treatments, and longer life. Insurance coverage is improving, but it’s still uneven.
What You Need to Ask Your Doctor
If you or someone you love has been diagnosed with a brain tumor, don’t accept a label without asking:
- What is the exact histological type?
- What are the IDH, 1p/19q, and MGMT results?
- What grade is it under WHO CNS5?
- Is this tumor likely to progress? When?
- What are the treatment options based on these results?
- Are there clinical trials I should consider?
Don’t be afraid to ask for a second opinion, especially from a major cancer center with a neuro-oncology team. The WHO CNS5 system is complex. Not every pathologist has mastered it yet.
And remember: a diagnosis isn’t a death sentence. Many people with Grade II tumors live 15-20 years. Even with glioblastoma, survival is improving. With vorasidenib and other targeted drugs on the horizon, the next five years could bring more progress than the last 20.
What does WHO CNS5 mean for brain tumor diagnosis?
WHO CNS5 is the 2021 update to the World Health Organization’s classification system for brain tumors. It requires both tissue analysis and molecular testing (like IDH mutation and 1p/19q codeletion) to assign a diagnosis and grade. This means two tumors that look the same under a microscope can be classified differently based on their genetics, leading to more accurate treatment plans.
Is a Grade 4 brain tumor always fatal?
Not always. Grade 4 glioblastoma, IDH-wildtype, has a median survival of about 14.6 months with standard treatment. But IDH-mutant Grade 4 astrocytomas have a median survival of 31 months. New drugs like vorasidenib are also extending survival for some patients. While still serious, outcomes are improving thanks to molecularly targeted therapies.
Can low-grade brain tumors become high-grade?
Yes. Low-grade tumors (Grade I and II) can transform into higher-grade cancers over time. This is called malignant transformation. That’s why regular MRI scans are critical-even if a tumor seems slow-growing. Monitoring helps catch changes early, when treatment is most effective.
What is vorasidenib, and who can take it?
Vorasidenib is an oral drug approved by the FDA in June 2023 for adults with Grade 2 IDH-mutant gliomas that can’t be fully removed by surgery. It blocks the abnormal protein made by the IDH mutation. In clinical trials, it doubled the time patients lived without their tumor growing compared to placebo-27.7 months versus 11.1 months.
Why does molecular testing cost so much?
Molecular testing requires specialized equipment and expertise to analyze DNA, RNA, and protein markers. Tests for IDH mutation, 1p/19q codeletion, and MGMT methylation each add cost. Labs also need to validate results for clinical use. While it can add $3,200-$5,800 to the diagnostic bill, this testing prevents ineffective treatments and helps choose the right therapy faster, saving money and time in the long run.
What’s Next for Brain Tumor Patients?
The future is moving toward precision medicine. Instead of treating all glioblastomas the same, doctors will soon match treatments to the specific mutations in your tumor. Clinical trials are testing drugs that target other mutations beyond IDH-like EGFR, BRAF, and H3K27M. Liquid biopsies may replace invasive surgeries for monitoring. And immunotherapy, once dismissed for brain tumors, is showing new promise.
One thing is clear: brain tumor care is no longer one-size-fits-all. The old system of grading by appearance alone is gone. The new system-based on biology, not just looks-is giving patients more time, more options, and more hope.




Comments (13)
Ryan Anderson
14 Nov 2025
Just read this whole thing cover to cover. Wow. I had no idea molecular markers changed everything. My uncle had a glioblastoma back in 2018 and they never tested for IDH or MGMT. He got the same treatment as everyone else. If they’d known then what we know now… 🤦♂️
Kevin Wagner
15 Nov 2025
Bro. This is the most hope I’ve felt about brain tumors in years. I used to think Grade 2 = death sentence. Now I see it’s more like a slow burn with a damn good chance of winning. Vorasidenib? That’s not a drug-it’s a lifeline. Someone needs to make a TikTok about this. 🚀
gent wood
16 Nov 2025
Excellent summary. The shift from morphology to molecular diagnostics is arguably the most significant advancement in neuro-oncology since the advent of MRI. The WHO CNS5 criteria, while complex, finally align classification with biological reality. I hope this becomes standard globally, not just in academic centers.
Scott Saleska
17 Nov 2025
Wait, so you're telling me if your tumor has IDH mutation, you're basically golden? That's it? No chemo? No radiation? Just sit back and wait? That's not how this works. I've seen people with IDH-mutant tumors die just as fast if they skip treatment. You're oversimplifying. This isn't a Netflix doc.
Jane Johnson
18 Nov 2025
Actually, the article is misleading. Grade 2 doesn't mean 'many years.' Many patients progress within 5. And vorasidenib? Only approved for non-resectable cases. The trial excluded elderly patients. This is cherry-picked optimism.
Peter Aultman
19 Nov 2025
man i just got diagnosed with a grade 2 astrocytoma last week and this post literally saved me from spiraling. i was googling 'brain tumor survival' and all i saw was 'death in 2 years' crap. this actually gave me a roadmap. thanks whoever wrote this
Dilip Patel
21 Nov 2025
USA always make everything sound like miracle drug. In India we dont even have MRI machine in small cities. Who cares about IDH mutation when you cant even get a neurologist? This is rich people medicine. Stop bragging.
Sean Hwang
21 Nov 2025
my cousin had a meningioma. they just watched it for 3 years. no surgery. no meds. just scans every 6 months. now it’s stable. so yeah sometimes the best treatment is doing nothing. this article nails that part.
Barry Sanders
23 Nov 2025
Wow. Another feel-good medical article. Where's the data on long-term cognitive decline? The 42% who thought Grade 2 meant 20% survival? That's not misinformation-that's people being smart enough to know the system is rigged. This reads like pharma PR.
Eleanora Keene
24 Nov 2025
As a nurse who’s worked in neuro-oncology for 12 years, I’ve seen families cry when they learn IDH status. Not because it’s bad-but because it’s *hopeful*. This isn’t just science. It’s human. I’ve held hands through every biopsy, every MRI, every wait. You’re not just treating a tumor. You’re treating a life. And now? We’re finally treating it right.
Chris Ashley
26 Nov 2025
so if you have a grade 4 but its idh mutant you dont die as fast? so like… its not as bad? but still bad? just… less bad? i dont get it
Ryan Anderson
27 Nov 2025
Exactly. It’s like comparing two car crashes. One’s a fender bender with airbags. The other’s a head-on with no seatbelt. Same outcome? No. Same car? Kinda. But the damage? Totally different. That’s IDH.
kshitij pandey
29 Nov 2025
from india i want to say thank you for writing this. my sister had a low grade tumor and we were told to wait. no one explained why. now i understand. i will share this with every family i know. knowledge is power. and hope is medicine too.