Generic vs Brand Drugs: Long-Term Safety Evidence Explained

When the FDAThe U.S. Food and Drug Administration is the federal agency responsible for protecting public health by ensuring the safety and efficacy of drugs. approves a generic drug, it means the medication contains the same active ingredient as the brand-name version. But does that guarantee identical safety over years of use? Real-world studies reveal surprising findings.
How Regulators Ensure Generic Safety
Before a generic drug hits shelves, it must prove bioequivalenceA regulatory standard ensuring generic drugs deliver the same amount of active ingredient at the same rate as the brand-name drug. to its brand counterpart. The FDA requires that 90% of the time, the generic's absorption falls within 80-125% of the brand's levels. This isn't just paperwork-over 2,000 studies submitted to the FDA between 1984 and 2018 show generics typically differ by just 3.5% in absorption. For most medications, this tiny gap means identical effects. But what about long-term risks? That's where things get complicated.
Contradictory Study Findings
Some studies show generics are safer than brands. A 2020 analysis of 17 million Austrians found people taking generic blood pressure medications had 53.8 deaths per 1,000 patient-years for brand drugs versus 30.2 deaths for generics. Survival rates were 77.8% for brand users versus 85.9% for generics. But other cases tell a different story. One patient switched from brand Ciproxin to generic ciprofloxacin and kept having infections. Only after returning to the brand did symptoms disappear. Another case involved generic levofloxacin causing persistent fever until switching back to Tavanic. These aren't rare-researchers estimate 30% of patients see no change after switching, 30% improve, and 30% get worse or stop taking the drug entirely.
Manufacturing Location Matters
Where a drug is made can change safety outcomes. A 2018 Ohio State study found Indian-made generic drugs had 54% higher severe adverse events (like hospitalizations) than U.S.-made versions. For ciprofloxacin specifically, Indian-made generics caused 62% more hospitalizations due to severe side effects. This wasn't about the drug itself-it was about manufacturing quality. The FDA's own database (FAERS) shows similar patterns for other drugs made overseas. If you're taking a generic, knowing where it's manufactured might matter more than you think.
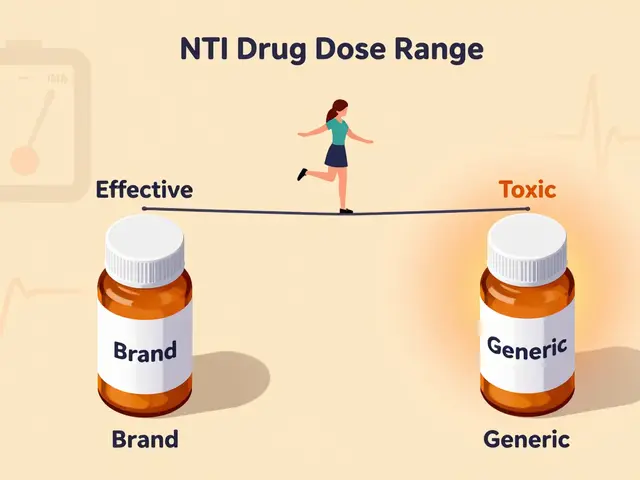
Narrow Therapeutic Index Drugs: When Small Differences Matter
Some drugs need precision. Medications like warfarin, levothyroxine, and lamotrigine have a narrow therapeutic indexA range where tiny changes in drug levels can cause serious side effects or treatment failure.. For levothyroxine, generics showed 12.3% higher fluctuations in thyroid hormone levels compared to brand Synthroid. One Reddit user reported seizures jumping from 1-2 per month to 8-10 after switching to generic lamotrigine. Only when they returned to brand Lamictal did their seizures stabilize. These cases highlight why doctors often stick with brand-name versions for critical medications.

Real-World Patient Experiences
What do patients actually experience? On r/pharmacy, a top post described seizures worsening after a generic switch. Meanwhile, PatientsLikeMe analyzed 3,842 hypertension patients: 78.4% saw no difference between brand and generic, 12.6% felt generics worked worse, and 9.0% said generics worked better. The FDA's adverse event database shows 1,247 reports of "generic drug ineffective" between 2018-2022-far more than brand-name reports-but this makes sense since generics make up 92% of prescriptions. The real question is: do these reports reflect actual safety issues or just more people using generics?
Current Consensus and Recommendations
Most experts agree generics are safe for the vast majority of drugs. The FDA, American Medical Association, and UCSF all state generics meet the same quality standards as brands. But they also acknowledge risks for "complex generics" like inhalers or injectables where bioequivalence testing may not capture long-term differences. In 2022, the FDA released new guidance for these tricky drugs. For now, doctors recommend sticking with brand-name versions for narrow therapeutic index medications, while generics remain a safe, affordable choice for most other conditions. With 92% of U.S. prescriptions filled as generics (but only 23% of drug spending), the trade-off between cost and safety continues to shape healthcare decisions worldwide.
Are generic drugs always as safe as brand-name drugs?
For most medications, yes. The FDA requires generics to meet strict bioequivalence standards, and studies show they work the same for 90% of drugs. However, for narrow therapeutic index drugs like warfarin or levothyroxine, small differences can cause problems. Always check with your doctor if you're taking one of these.
Can switching from brand to generic cause side effects?
Yes, sometimes. While most people don't experience issues, studies show 30% of patients report side effects or reduced effectiveness after switching. This is especially true for drugs like ciprofloxacin or lamotrigine. If you notice new symptoms after a switch, contact your doctor immediately-stopping the generic and returning to the brand often resolves the problem.
Why do some generic drugs come from India?
Most generic drugs are manufactured overseas because production costs are lower. India supplies about 40% of U.S. generics, but a 2018 study found Indian-made generics had 54% more severe adverse events than U.S.-made versions. This doesn't mean all Indian-made drugs are unsafe-it highlights the importance of FDA inspections and quality control checks on foreign facilities.
What is a narrow therapeutic index drug?
These are medications where a small change in dosage can cause serious harm. Examples include warfarin (blood thinner), levothyroxine (thyroid medication), and lamotrigine (seizure treatment). For these drugs, even tiny differences between brand and generic versions can lead to treatment failure or dangerous side effects. Always verify with your pharmacist whether your prescription has a narrow therapeutic index.
Should I avoid generic drugs entirely?
No. Generics are safe and effective for most conditions, saving patients billions annually. The FDA approves over 90% of generics without safety concerns. Only avoid them for specific drugs with narrow therapeutic indexes or if you've personally experienced issues after switching. Always discuss concerns with your doctor-they can help you weigh the cost savings against potential risks for your situation.




Comments (9)
Jenna Elliott
6 Feb 2026
US made generics are the only safe choice. Indian manufacturing is a joke. FDA data shows 54% more adverse events. Time to stop importing unsafe drugs.
Sam Salameh
7 Feb 2026
Agree with Jenna! US manufacturing standards are top notch. FDA inspections ensure quality. Why risk it with foreign generics? Safety first.
Dr. Sara Harowitz
9 Feb 2026
I must stress-the FDA's FAERS database reveals a staggering 54% increase in severe adverse events for Indian-made generics! This is not a trivial statistic-it's a clear warning! We must demand stricter oversight and prioritize domestic production! It's a matter of public health!
Carol Woulfe
11 Feb 2026
The FDA's approval process for generics is fundamentally flawed.
They rely on bioequivalence standards that ignore long-term effects.
This is a deliberate strategy by pharmaceutical conglomerates to maximize profits at the expense of patient safety.
The data speaks for itself-higher adverse events from overseas manufacturing.
The system is rigged.
It's not just about the drugs themselves but the entire regulatory framework.
The FDA's own database shows alarming trends.
They prioritize cost-cutting over patient well-being.
This is a clear case of corporate influence in government agencies.
We need to demand transparency and accountability.
The public deserves better than this.
It's time to expose the truth.
This isn't just about drugs-it's about the integrity of our healthcare system.
Every time we allow this to continue, lives are put at risk.
We must act now before more people suffer.
Georgeana Chantie
13 Feb 2026
Actually most studies show generics are just as safe. FDA requires rigorous testing. The 54% figure is misleading-it's not about the drug itself but manufacturing practices. Many Indian facilities meet FDA standards. This fearmongering is unnecessary 😏
Lisa Scott
14 Feb 2026
This is a public health crisis.
Kieran Griffiths
15 Feb 2026
Lisa, I understand your concerns but the FDA has strict protocols for foreign facilities. Many Indian manufacturers meet US standards. The 54% figure is from a specific study-context matters. Not all Indian generics are problematic. Always check the manufacturer and consult your pharmacist.
Tehya Wilson
16 Feb 2026
Whilst the data indicates disparities in adverse events between manufacturing origins, blanket condemnation is unwarranted. The FDA's oversight mechanisms are robust. A nuanced approach is required-specifically targeting substandard facilities rather than entire countries. This is a complex issue requiring evidence-based policy.
anjar maike
17 Feb 2026
Interesting points. But what about the 78.4% of hypertension patients who saw no difference? Maybe the problem is not generics but specific manufacturers. Also why do some countries have better quality control? 🤔