Preventive Measures for Building Resilient Pharmaceutical Supply Chains
When a life-saving antibiotic disappears from pharmacy shelves, it’s not a glitch-it’s a systemic failure. In 2024, over 300 critical medications faced shortages in the U.S. alone, from injectable painkillers to chemotherapy drugs. These aren’t random events. They’re the predictable result of supply chains built for efficiency, not endurance. The truth is simple: if you want to stop drug shortages, you have to rebuild how medicines are made, moved, and stored. It’s not about blame. It’s about building resilience.
Why Your Medicine Might Not Be There Tomorrow
Most people assume drug shortages happen because of bad weather or factory fires. But the real problem runs deeper. About 80% of the active ingredients in U.S. medicines come from overseas, mostly from China and India. That’s not just a number-it’s a vulnerability. A single factory shutdown in Hyderabad or a trade dispute in Shanghai can ripple across hospitals from Chicago to Cleveland. In 2023, the FDA confirmed that 40% of finished drug products sold in the U.S. rely on foreign manufacturing. For sterile injectables, the number drops to just 12% made domestically. Antibiotics? Only 17%. These aren’t backup systems. They’re single points of failure. The pandemic didn’t create this problem. It exposed it. Before 2020, companies chased the cheapest production costs, cutting inventory to the bone. They trusted global logistics to deliver just-in-time. But when borders closed and shipping slowed, there was no buffer. No safety net. Hospitals ran out of IV bags. Cancer patients waited weeks for their next dose. That’s not supply chain management. That’s gambling with public health.What Resilience Actually Means in Practice
Resilience isn’t a buzzword. It’s a set of actions. The U.S. Department of Health and Human Services defines it clearly: the ability to anticipate, prepare for, respond to, and recover from disruptions while keeping critical medicines flowing. That means three things: preparedness, response, and recovery. Preparedness starts with knowing where your ingredients come from-not just the first supplier, but the 12th tier down. Leading companies now map every link in their supply chain, from raw chemicals to finished pills. They ask: What if this factory in Guangdong shuts down? What if the port in Los Angeles backs up for two months? What if a new regulation blocks exports from India? They don’t guess. They simulate. They run drills. They build scenarios. Response means having options ready. That’s where dual-sourcing comes in. Instead of relying on one supplier for a key API, top firms now use two or three, spread across different regions. For the most critical drugs, they maintain 60 to 90 days of inventory-something most companies haven’t done since the 1990s. This isn’t waste. It’s insurance. And it works. Companies that implemented these strategies saw 23% higher continuity during disruptions, avoiding an average of $14.7 million in lost revenue per event. Recovery is about bouncing back faster. That’s where new manufacturing tech comes in. Traditional batch production takes months to set up and adjust. Continuous manufacturing-like a chemical assembly line-can be reconfigured in weeks. It uses 30% less space, 25% less energy, and cuts waste by 15-20%. The FDA approved just 12 continuous manufacturing facilities as of mid-2025. That’s tiny compared to the 10,000+ batch plants. But the trend is clear: by 2027, nearly half of new manufacturing capacity will use this tech.
The Real Cost of Not Acting
Some say resilience is too expensive. They’re wrong. The cost of doing nothing is higher. Building buffer stocks, diversifying suppliers, upgrading facilities-these cost 8-12% more on the cost of goods sold. But when a shortage hits, hospitals pay more for emergency shipments. Patients miss treatments. Mortality rises. The economic toll isn’t just in lost sales-it’s in emergency care, extended hospital stays, and lost productivity. Companies that invest in resilience see a 1.8x return on investment within three years, according to PwC. And it’s not just about money. The U.S. Department of Defense calls pharmaceutical supply chain gaps a critical national security risk. If a conflict or cyberattack cuts off access to blood thinners or anesthetics, the consequences aren’t theoretical. They’re deadly. That’s why the White House launched the Strategic Active Pharmaceutical Ingredients Reserve in August 2025. It’s a government-backed stockpile targeting 150 essential medicines, aiming for 90 days of coverage by 2027. This isn’t socialism. It’s risk management.Where the Industry Is Heading
The old model-global, lean, ultra-cheap-is dead. The new model is regional, diversified, and slightly more expensive. North American companies have already cut China-sourced APIs from 38% in 2022 to 29% in 2025. Domestic production has climbed from 22% to 28%. That’s progress, but not enough. The U.S. still produces less than a third of its essential medicine ingredients. The future lies in balance. You don’t need every pill made in America. But you do need to make sure the most critical ones are. Sterile injectables. Insulin. Chemotherapy drugs. These should be produced in multiple regions: North America, Europe, Southeast Asia, and Latin America. That way, if one region fails, others can step in. Technology is accelerating this shift. AI tools now predict supply disruptions with 85-90% accuracy up to 90 days ahead. Blockchain systems track every vial from factory to patient, cutting counterfeit drugs by 70%. These aren’t sci-fi tools-they’re being piloted now. But tech alone won’t fix this. People will. The industry faces a shortage of 250,000 skilled manufacturing workers by 2027. Training programs, apprenticeships, and better pay are as important as new factories.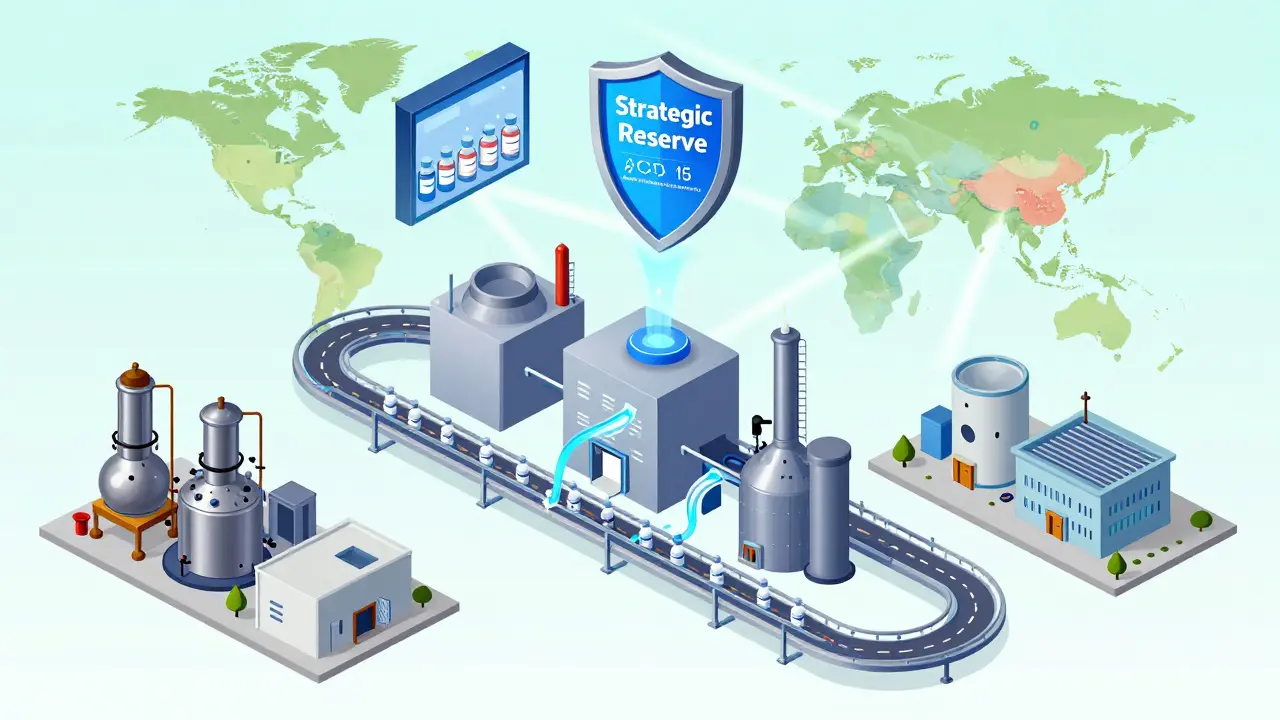
What Needs to Happen Now
If you’re in pharma, here’s what you need to do:- Map your supply chain down to Tier 5. Know who makes the raw chemicals, not just the final product.
- Dual-source at least 70% of your critical APIs. Don’t rely on one country or one plant.
- Build 60-90 day buffer stocks for life-saving drugs. This isn’t hoarding-it’s responsibility.
- Invest in continuous manufacturing where it makes sense. Even a small pilot line can cut lead times by months.
- Train your team on disruption scenarios. Run quarterly drills. Treat it like a fire evacuation plan.
- Expand the Strategic Active Pharmaceutical Ingredients Reserve to cover 200+ drugs by 2028.
- Offer tax credits for companies that invest in domestic API production-especially for sterile injectables and antibiotics.
- Harmonize global manufacturing standards. Right now, 65% of regulations don’t align between the U.S., EU, and Japan. That slows innovation.
- Fund workforce training programs. No factory runs on software alone.
The Bottom Line
Drug shortages aren’t accidents. They’re design flaws. We built supply chains to be cheap, not safe. Now we’re paying the price-in delayed treatments, scared patients, and stressed nurses. The fix isn’t magic. It’s methodical. It’s investing in redundancy. It’s accepting that resilience costs more upfront but saves lives later. It’s making sure that when a crisis hits, your medicine isn’t just available-it’s guaranteed. The next time you hear about a drug shortage, don’t ask who’s to blame. Ask: What are we doing to make sure this never happens again?What causes pharmaceutical supply chain disruptions?
The biggest causes are geopolitical tensions, natural disasters, regulatory changes, and single-source dependencies. Over 80% of active pharmaceutical ingredients (APIs) come from just two countries-China and India. A factory shutdown, export ban, or port delay in either country can trigger nationwide shortages. Other causes include raw material shortages, quality control failures, and labor strikes in manufacturing hubs.
How does buffer stock help prevent drug shortages?
Buffer stock is a safety reserve of critical medicines kept on hand to cover unexpected disruptions. For essential drugs like insulin or antibiotics, maintaining 60 to 90 days of inventory gives hospitals and distributors time to find alternative suppliers or ramp up production. This prevents panic buying and ensures patients don’t go without treatment during a crisis. Companies that use buffer stocks report 30-40% fewer shortage events.
Is domestic manufacturing the answer to drug shortages?
Not alone. While increasing U.S. production helps-domestic API output rose from 22% to 28% between 2022 and 2025-it’s not enough. Building all manufacturing domestically would raise drug costs by 20-30% and create new risks, like over-reliance on a single region. The best approach is regional diversification: producing critical drugs in multiple zones-North America, Europe, Southeast Asia-so no single event can cut off supply.
What role does AI play in preventing supply chain breakdowns?
AI predicts disruptions before they happen. By analyzing global shipping data, weather patterns, political events, and supplier performance, AI tools can forecast potential shortages up to 90 days in advance with 85-90% accuracy. This lets companies reorder materials early, reroute shipments, or shift production. Companies using AI for supply chain monitoring reduce response time by 50% and cut inventory waste by 20%.
Why aren’t more companies investing in resilient supply chains?
The main barriers are cost, complexity, and short-term thinking. Building buffer stocks, dual-sourcing, and upgrading to continuous manufacturing require major upfront investment-$50 million to $150 million per facility. Many companies, especially smaller ones, don’t have the capital. Others lack integrated data systems to track suppliers across 12+ tiers. And because disruptions are rare, leadership often sees resilience as an unnecessary expense-until a shortage hits and it’s too late.
How long does it take to build a resilient supply chain?
It’s not a one-time project-it’s an ongoing process. A full vulnerability assessment takes 3-6 months. Strategic planning and supplier diversification take another 6-12 months. Implementing new manufacturing tech like continuous production can take 18-24 months. But you can start seeing benefits within 6 months by simply mapping your top 10 critical suppliers and adding buffer stock for your most essential drugs.
What’s the difference between continuous manufacturing and batch production?
Batch production makes medicine in separate lots-like baking cookies in batches. It’s slow, requires big factories, and takes months to adjust. Continuous manufacturing is like a流水线 (assembly line) where ingredients flow in and finished drugs come out nonstop. It’s faster, uses 30-40% less space, cuts energy use by 20-25%, and reduces waste by 15-20%. The FDA has approved only 12 continuous facilities so far, but that number is expected to grow rapidly after new 2025 guidance cut approval times from 3 years to under 18 months.





Comments (13)
Roisin Kelly
20 Jan 2026
Of course it's a systemic failure. Who do you think controls the global pharma supply? Big Pharma + the CIA + China. They let shortages happen to keep prices high and people scared. You think they care if grandma dies without her antibiotics? Nah. They're busy counting their billions while you're begging for a prescription.
lokesh prasanth
20 Jan 2026
China makes 80% APIs? So we're addicted to red flags. We built this. We chose cheap over safe. Now cry about it. Simple.
MAHENDRA MEGHWAL
22 Jan 2026
The structural vulnerabilities outlined in this post are both profound and alarming. One must recognize that the current paradigm of globalized pharmaceutical manufacturing, while economically efficient, has systematically eroded national sovereignty over essential health commodities. The absence of redundancy is not negligence-it is a calculated risk imposed upon the public without informed consent.
Dee Monroe
23 Jan 2026
I keep thinking about how we treat medicine like it's just another product, like smartphones or sneakers. We want it cheap, fast, and disposable-but when it's your kid's asthma inhaler or your mom's chemo, it stops being a commodity and becomes a lifeline. We're not just talking about supply chains here, we're talking about how we value human life. And if we're honest, we've been treating it like a spreadsheet line item for decades. It's time we started treating it like what it is: sacred.
Sangeeta Isaac
25 Jan 2026
So let me get this straight-we spent 20 years chasing the cheapest pills possible, now we're shocked when the pills vanish? 🤦♀️ Meanwhile, the FDA approved 12 continuous manufacturing plants... out of 10,000? Bro. We're running a hospital with a flip phone and wondering why the Wi-Fi is down. Also, I just got my insulin from a warehouse in Mexico. So much for 'domestic production'.
Philip Williams
25 Jan 2026
The data presented here is compelling and requires immediate action. The economic argument for resilience is not merely prudent-it is imperative. The return on investment of 1.8x within three years, coupled with the national security implications, renders inaction indefensible. Stakeholders across industry and government must align on standardized metrics for supply chain resilience.
Jerry Rodrigues
27 Jan 2026
I get the urgency but I also think we're ignoring the human side. The workers in those Indian and Chinese factories? They're not faceless cogs. They're moms and dads trying to feed their kids. If we just pull all production home, we're not fixing the system-we're just moving the pain. We need fairness, not just security.
Barbara Mahone
27 Jan 2026
The notion that resilience requires redundancy is not novel, yet its implementation remains fragmented. Regulatory harmonization between the U.S., EU, and Japan would reduce compliance burdens and accelerate adoption of best practices. A unified framework, grounded in science and transparency, would serve public health far more effectively than isolated national initiatives.
Amber Lane
29 Jan 2026
Buffer stocks aren't hoarding. They're common sense.
Gerard Jordan
29 Jan 2026
AI predicting shortages 90 days out? 🤯 That's wild. But also... why aren't we using this tech to track which hospitals are running low in real time? Like, if my local pharmacy's insulin stock is at 10%, why can't I get a push notification? We got the tools. We just ain't using 'em right. 🙏💊
Samuel Mendoza
31 Jan 2026
Resilience? More like socialism with a pharma label. Let the market fix it. If drugs are scarce, prices rise. That's how it works. Stop forcing companies to waste money on 'buffer stocks'.
Melanie Pearson
2 Feb 2026
Let me be clear: the U.S. cannot rely on foreign nations-especially China-for life-saving medications. This is not an economic issue. It is treasonous negligence. Domestic production must be mandatory, not optional. We are a superpower. We should be manufacturing 100% of our essential drugs. Period.
Uju Megafu
4 Feb 2026
So let me get this straight-you want to fix drug shortages by building more factories in the US? Meanwhile, your own government gives billions in subsidies to Big Pharma while nurses work 16-hour shifts and patients die because they can't afford their meds? This isn't about supply chains. It's about greed. And you're just rearranging deck chairs on the Titanic while the real crisis is the profit motive.