Autoimmune Disease Monitoring: Lab Markers, Imaging, and Visits
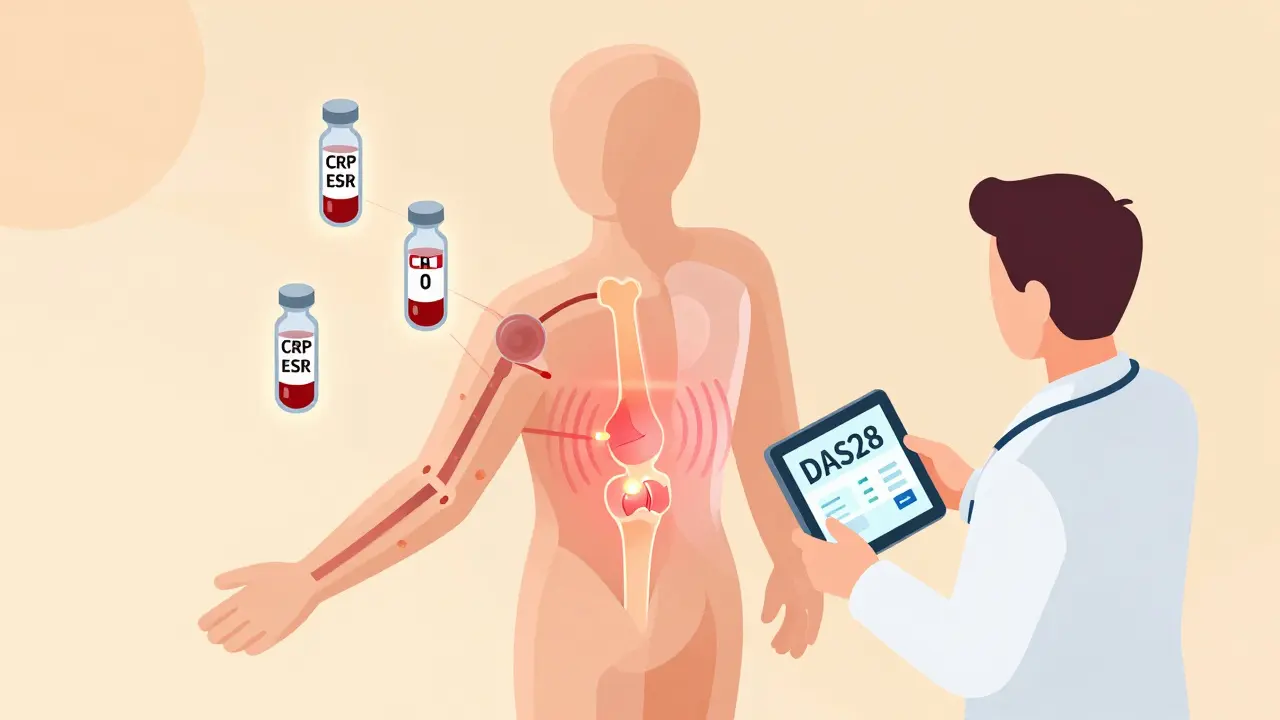
Managing an autoimmune disease isn’t just about taking medication. It’s about staying one step ahead of your body’s misdirected immune response. Without regular monitoring, flares can sneak up, organs can get damaged, and treatment can fall out of sync with what your body actually needs. The good news? There’s a clear, evidence-backed system for tracking autoimmune diseases using three key tools: lab markers, imaging, and scheduled clinical visits.
Lab Markers: What Blood Tests Actually Tell You
Lab tests are the backbone of autoimmune monitoring, but not all tests are created equal. Many patients think a positive ANA test means their disease is active - but that’s a myth. The antinuclear antibody (ANA) test is a screening tool, not a tracker. About 20% of healthy people test positive for ANA. Once you’ve been diagnosed, serial ANA tests won’t tell you if you’re in a flare or in remission. They stay positive even when the disease is quiet.
What you should track are the real-time inflammation signals. C-reactive protein (CRP) is one of them. Levels above 3.0 mg/L suggest active inflammation. Erythrocyte sedimentation rate (ESR) is another - over 20 mm/hr in women and 15 mm/hr in men points to ongoing immune activity. These aren’t perfect, but they’re your best early warning system.
Then there are the disease-specific autoantibodies. If you have lupus, anti-dsDNA antibodies are critical. They’re found in 60-70% of cases and rise when kidney involvement (lupus nephritis) is worsening. Their specificity is 95%, meaning if they spike, it’s likely your lupus is acting up. Complement levels (C3 and C4) drop during active disease - so falling levels mean trouble, even if CRP is normal.
For Sjögren’s syndrome or scleroderma, the ENA panel matters. SS-A antibodies show up in over 80% of Sjögren’s cases. Scl-70 is linked to systemic sclerosis, and Jo-1 appears in about 17% of polymyositis patients. These aren’t checked every visit - only when needed to confirm subtype or assess organ risk.
ELISA is still the most common method for detecting these antibodies because it’s cheap and widely available. But it has limits. Results can vary depending on the lab, the kit brand, or even the technician. Multiplex assays and newer technologies like CyTOF (mass cytometry) are changing the game. CyTOF can measure up to 50 immune cell markers at once, giving doctors a detailed snapshot of your immune system’s behavior - not just one antibody level. It’s not in every clinic yet, but it’s becoming standard in top rheumatology centers.
Imaging: Seeing What Blood Tests Can’t
Lab tests tell you something’s wrong. Imaging tells you where and how bad. Many autoimmune diseases attack joints, lungs, kidneys, or skin - organs you can’t feel changing until damage is done. That’s why imaging is non-negotiable.
Magnetic resonance imaging (MRI) is the gold standard for early inflammation. It picks up swelling in joints, tendons, and even the brain long before X-rays show damage. Newer contrast agents using nanotechnology are safer than old gadolinium-based dyes and give clearer pictures of tissue inflammation.
Ultrasound is quietly revolutionizing rheumatoid arthritis and lupus monitoring. With acoustically active microbubbles, it measures blood flow in inflamed joints. Studies show it’s 85% accurate at detecting synovitis - the inflammation that leads to joint erosion. It’s faster, cheaper, and radiation-free compared to CT or PET scans.
Positron Emission Tomography (PET) scans are no longer just for cancer. New tracers can tag immune cells, letting doctors see where T-cells are gathering in the body. One recent study tracked immune activity in lupus patients using radiolabeled antibodies - showing hotspots of inflammation in the spleen and lymph nodes that blood tests missed entirely.
SPECT scans use radioactive peptides to highlight molecular activity at inflammation sites. They’re not routine, but they’re powerful for complex cases, like vasculitis or unexplained organ pain. CT scans help evaluate structural damage - lung scarring in scleroderma, bone erosion in RA, or kidney size changes in lupus.
Here’s the key: imaging isn’t about finding something new. It’s about catching changes early. A joint that looks normal on X-ray might already be inflamed on ultrasound. A kidney that looks fine on CT might have early scarring only visible on MRI. That’s why imaging is paired with lab work - they fill each other’s blind spots.
How Often Should You See Your Doctor?
There’s no one-size-fits-all schedule. But there are clear guidelines. The American College of Rheumatology and EULAR agree: monitoring must be structured, not reactive.
After diagnosis, visits are frequent - every 4 to 6 weeks - until your disease is under control. That’s when your doctor adjusts meds, checks for side effects, and makes sure you’re not heading into a flare.
Once you’re stable, visits drop to every 3 to 4 months. But “stable” doesn’t mean “done.” You still need a full checkup at least twice a year, including blood work, physical exam, and a conversation about how you’re feeling.
For high-risk patients - those with kidney, lung, or neurological involvement - quarterly visits with imaging are recommended. For mild cases, like early-stage psoriatic arthritis with no organ damage, every 6 to 12 months may be enough.
Doctors use scoring systems to track progress. DAS28 for rheumatoid arthritis, SLEDAI for lupus - these combine joint counts, lab values, and patient reports into one number. A rising score means your treatment needs tweaking. A falling score means it’s working.
And here’s something patients often overlook: your own symptoms matter just as much as the numbers. If you’re exhausted, your joints feel stiff in the morning, or your skin is breaking out - tell your doctor. These aren’t “just symptoms.” They’re data points. Studies show that 63% of flares show clear clinical signs before any lab or imaging change appears.
The Real Cost of Skipping Monitoring
Skipping visits or skipping tests sounds harmless - until it’s not. A 2021 study in the Journal of Autoimmunity found that patients with structured monitoring had 37% fewer hospitalizations and 28% less long-term disability than those who only went when they felt bad.
Why? Because autoimmune diseases don’t wait. Lupus nephritis can destroy kidney function in months. Vasculitis can cause nerve damage before you feel numbness. RA can erode joints silently. Monitoring isn’t about being paranoid. It’s about catching problems before they become emergencies.
But access is uneven. Only 48% of Medicaid patients get recommended monitoring, compared to 83% of those with private insurance. Imaging and advanced lab tests are expensive. Insurance often denies PET scans or CyTOF unless you’ve failed three other treatments. That’s not just unfair - it’s dangerous.

What’s Next? Wearables, AI, and Digital Platforms
The future of autoimmune monitoring is already here. In 2023, the FDA approved the first integrated digital platform for autoimmune disease tracking: AutoimmuneTrack. It pulls together lab results, wearable sensor data (like heart rate variability and sleep patterns), patient-reported symptoms, and AI analysis.
In a trial with over 2,300 patients, it predicted flares 14 days in advance with 76% accuracy. Users had 29% fewer ER visits. Wearables that analyze interstitial fluid for CRP-like markers are showing 89% correlation with traditional blood tests. AI can spot patterns in your history - like how your ESR spikes every time you skip sleep - and warn you before you feel sick.
This isn’t science fiction. It’s the new standard. The global market for autoimmune monitoring is now $12.7 billion, and it’s growing fast. But technology won’t fix access gaps. If you’re struggling to afford tests or visits, talk to your doctor. There are patient assistance programs, sliding-scale labs, and clinical trials that offer free monitoring.
What You Can Do Today
You don’t need a high-tech platform to take control. Start here:
- Keep a simple symptom log: fatigue, joint pain, rashes, fever - note when they start and how long they last.
- Ask your doctor: “Which lab markers should I track monthly? Which ones are just for diagnosis?”
- Request imaging if you have persistent pain, even if labs are normal.
- Don’t skip visits because you “feel fine.” Flares don’t always announce themselves.
- If insurance denies a test, ask for a letter of medical necessity. Many are approved on appeal.
Autoimmune disease isn’t a one-time diagnosis. It’s a lifelong conversation with your body. The right monitoring turns that conversation into a partnership - one that helps you live well, not just survive.
Is ANA testing useful for monitoring autoimmune disease activity?
No, ANA testing is not useful for monitoring disease activity. While it’s a key diagnostic tool - positive in 95% of systemic lupus cases - ANA levels remain positive even during remission. Relying on ANA to track flares can lead to false reassurance or unnecessary treatment changes. Instead, monitor CRP, ESR, anti-dsDNA antibodies, and complement levels (C3/C4), which fluctuate with actual disease activity.
How often should I get imaging for my autoimmune condition?
There’s no universal schedule. Imaging frequency depends on your disease, severity, and symptoms. For rheumatoid arthritis, ultrasound every 6-12 months is common if stable. For lupus with kidney risk, MRI or CT may be done annually. If you have new or worsening pain, imaging should be requested immediately - don’t wait for your next routine visit. Always ask: “Could imaging show something blood tests can’t right now?”
Why do my lab results keep changing even when I feel fine?
Autoimmune diseases don’t always match how you feel. Your immune system can be quietly active - even if you’re not having symptoms. CRP or ESR might rise due to stress, infection, or sleep loss. Anti-dsDNA can fluctuate with hormonal changes. That’s why doctors look at trends over time, not single numbers. One high value doesn’t mean a flare. Three high values in a row, with symptoms, do.
Can I rely on wearable devices to replace lab tests?
Not yet. Wearables that track sleep, heart rate, or interstitial fluid biomarkers are promising - some show 89% correlation with CRP levels. But they’re supplements, not replacements. Labs still measure specific antibodies, organ function, and inflammation markers that wearables can’t detect. Use wearables to spot patterns and alert you to potential issues, but always confirm with blood tests and your doctor.
What if I can’t afford recommended tests or visits?
You’re not alone. Many patients face insurance denials or high out-of-pocket costs. Ask your rheumatologist for help - many labs offer sliding-scale fees. Patient advocacy groups like AARDA or the Lupus Foundation provide grants for testing. Some clinical trials offer free monitoring. Don’t skip care because of cost. Talk to your care team. There are always options, even if they’re not obvious.





Comments (9)
Meghan O'Shaughnessy
18 Dec 2025
Been living with lupus for 12 years and honestly, the ANA myth is everywhere. My first rheum told me to stop getting it checked - said it was like checking your blood type every month. CRP and C3/C4? That’s what I track. My last flare? CRP spiked to 8.2, ANA was still 1:640 like it’s been since 2012. Funny how we cling to old info when the science moved on.
Evelyn Vélez Mejía
19 Dec 2025
The epistemological scaffolding underpinning autoimmune monitoring reveals a profound dissonance between clinical utility and patient perception. The ANA test, as a diagnostic artifact, has been ontologically reified into a proxy for disease activity - a semiotic error of monumental proportion. We mistake the map for the territory, the symbol for the substance. CRP and ESR, though imperfect, are dynamic indicators of systemic inflammation; they are not mere artifacts but phenomenological signatures of immune dysregulation. To conflate static serological markers with kinetic physiological states is not merely inaccurate - it is a form of epistemic negligence.
Nishant Desae
20 Dec 2025
hey guys i just wanted to say i really appreciate this post. i’ve been dealing with RA since 2020 and honestly i thought my doctor was just being extra when she asked for ultrasound every 6 months. but last year when my joints felt fine but the ultrasound showed synovitis? i got my meds adjusted before i had any damage. now i’m like a total nerd about tracking my numbers. i even have a little notebook where i write down how tired i feel on a scale of 1-10. and yeah sometimes the labs are weird when i’m stressed or slept bad but that’s why we look at trends. also big shoutout to my rheum doc for always listening when i say ‘i don’t feel right’ even if my crp is normal. you guys are doing great work.
Kaylee Esdale
21 Dec 2025
Wearables are cool but dont trade blood tests for a fancy watch. I got mine last year and it told me i was ‘stressed’ for 3 days straight. Turned out i had a UTI. Labs don’t lie. Also if your doc says ‘ANA is high so you’re flaring’ - walk out. Find someone who knows what they’re doing.
CAROL MUTISO
22 Dec 2025
Oh honey. You mean to tell me that after spending $20,000 on biologics and 17 different blood tests, the one thing we’ve been chasing for years - the holy grail of ‘is my disease active?’ - is actually just… CRP and C3? And the ANA? Just a party favor from 1998? I feel like I’ve been paying for a luxury spa package that only gives me a towel and a lemon wedge.
Virginia Seitz
23 Dec 2025
My doctor won’t order a PET scan unless I’m dying 😭 But I’ve been having unexplained fever for 4 months. They say ‘wait till labs change.’ What if they never do? 🤷♀️
Michael Whitaker
24 Dec 2025
While I appreciate the general sentiment, I must note that the assertion regarding CyTOF being 'standard in top rheumatology centers' is an overstatement. In reality, only a handful of academic institutions - perhaps fewer than 12 globally - possess the requisite infrastructure and funding to deploy such technology routinely. To suggest otherwise is to misrepresent the current state of clinical accessibility and, frankly, indulges in a form of technocratic wishful thinking that does a disservice to patients in underserved communities.
Brooks Beveridge
26 Dec 2025
Hey Virginia - I feel you. My sister had the same thing. She finally got a PET scan after begging for 9 months. Turned out she had early vasculitis in her lungs. No symptoms. No lab changes. Just a hot spot on the scan. Don’t give up. Ask for a second opinion. Write a letter. Call the insurance ombudsman. You’re not being dramatic - you’re being smart. And if your doc rolls their eyes? Find a new one. Your body deserves better.
Anu radha
27 Dec 2025
My mom has scleroderma. She never got the ENA panel until she had trouble swallowing. Now she gets it every 6 months. I wish we knew sooner. Thanks for explaining this.