Category: Pharmacy - Page 2
Specialty pharmacies dispense generic specialty drugs under the same strict rules as brand-name versions. Learn why cost doesn’t change the process - and how providers ensure safe, consistent care.
Bioavailability studies ensure generic drugs work like brand-name versions by measuring how much and how fast the active ingredient enters the bloodstream. The FDA uses strict criteria to approve generics without full clinical trials.
Understand what happens during an FDA inspection of generic drug manufacturing facilities, from Pre-Approval Inspections to FDA 483 responses and how to maintain CGMP compliance in 2025.
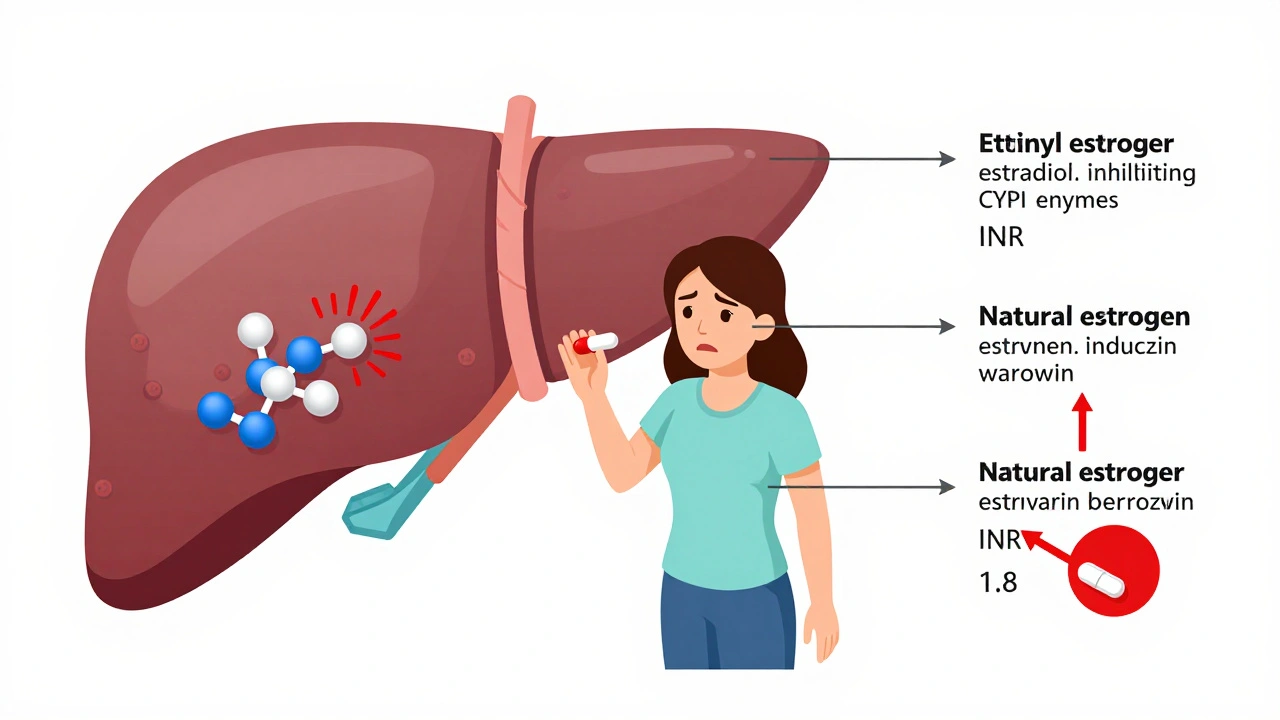
Estrogen can raise or lower your INR when you're on warfarin, increasing bleeding or clotting risk. Learn how birth control, HRT, and your genes affect warfarin dosing and what steps to take to stay safe.
Pharmacists face real challenges when substituting brand-name drugs with generics-patient mistrust, unclear doctor guidance, and time constraints. Here’s what really happens behind the counter and how to make it work.
Black box warnings are the FDA's strongest safety alerts for prescription drugs, signaling life-threatening risks. Learn what they mean, how they're decided, and what you should do if your medication has one.
Learn the real difference between medication side effects and adverse drug reactions-why it matters for your safety, how to spot them, and what to do when you experience a reaction.
Most drugs don't have authorized generics because they're a strategic tool for brand manufacturers, not a benefit for patients. Learn why only a small fraction of medications offer this cheaper option - and how it affects your prescription costs.
Hospital pharmacies are bearing the brunt of a growing crisis: sterile injectable drug shortages. With anesthetics, chemotherapy, and IV fluids running out, patient care is being delayed, surgeries postponed, and ethical dilemmas mounting. The system is broken - and the fix isn't coming soon.
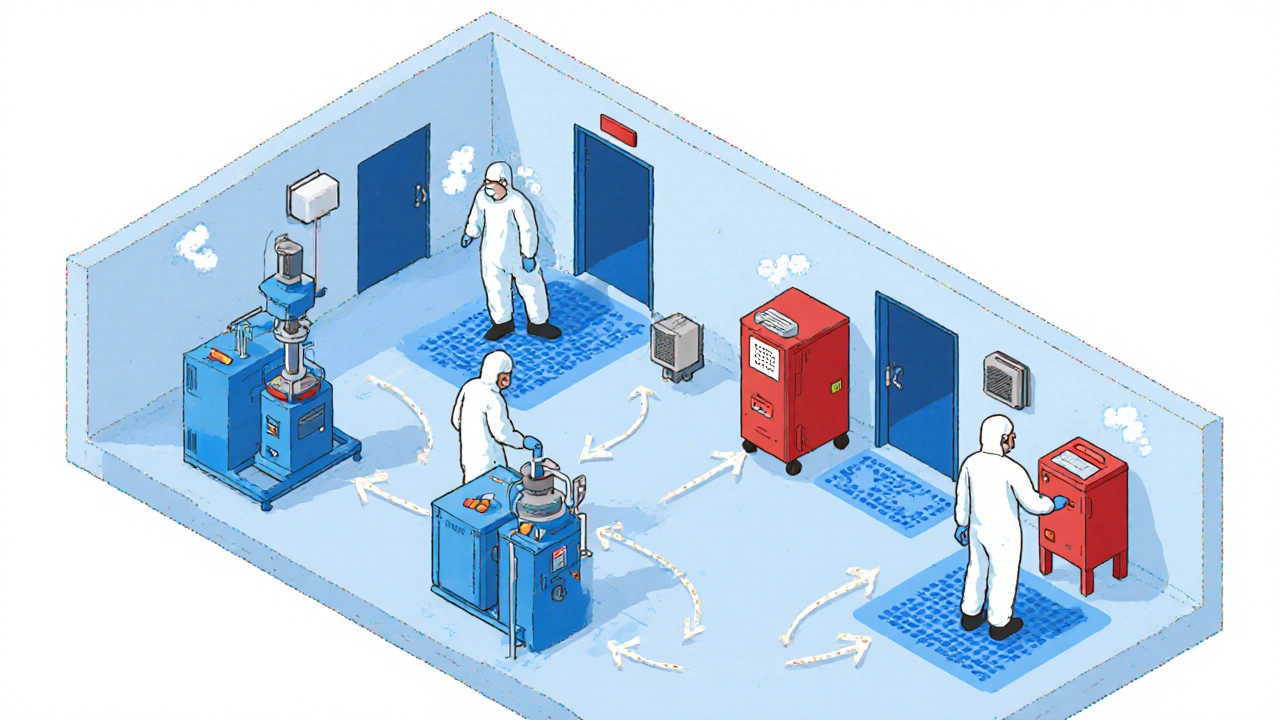
Contamination controls in generic drug manufacturing prevent dangerous adulteration through strict cleaning protocols, cleanroom design, and real-time monitoring. Learn how facilities stay compliant and avoid costly recalls.
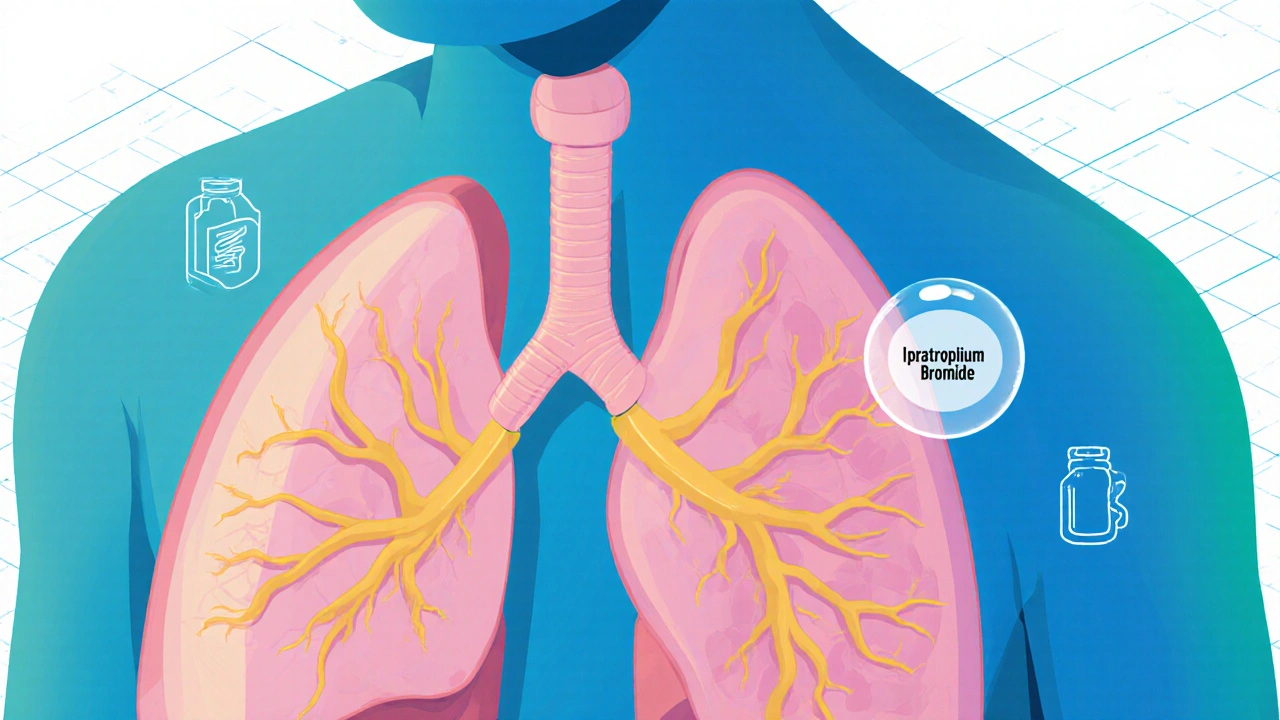
Ipratropium bromide is a trusted bronchodilator used to open airways in COPD and some asthma cases. It works slowly but steadily, offering relief without the heart side effects of other inhalers. Safe for long-term use, it’s a cornerstone of daily breathing management for millions.
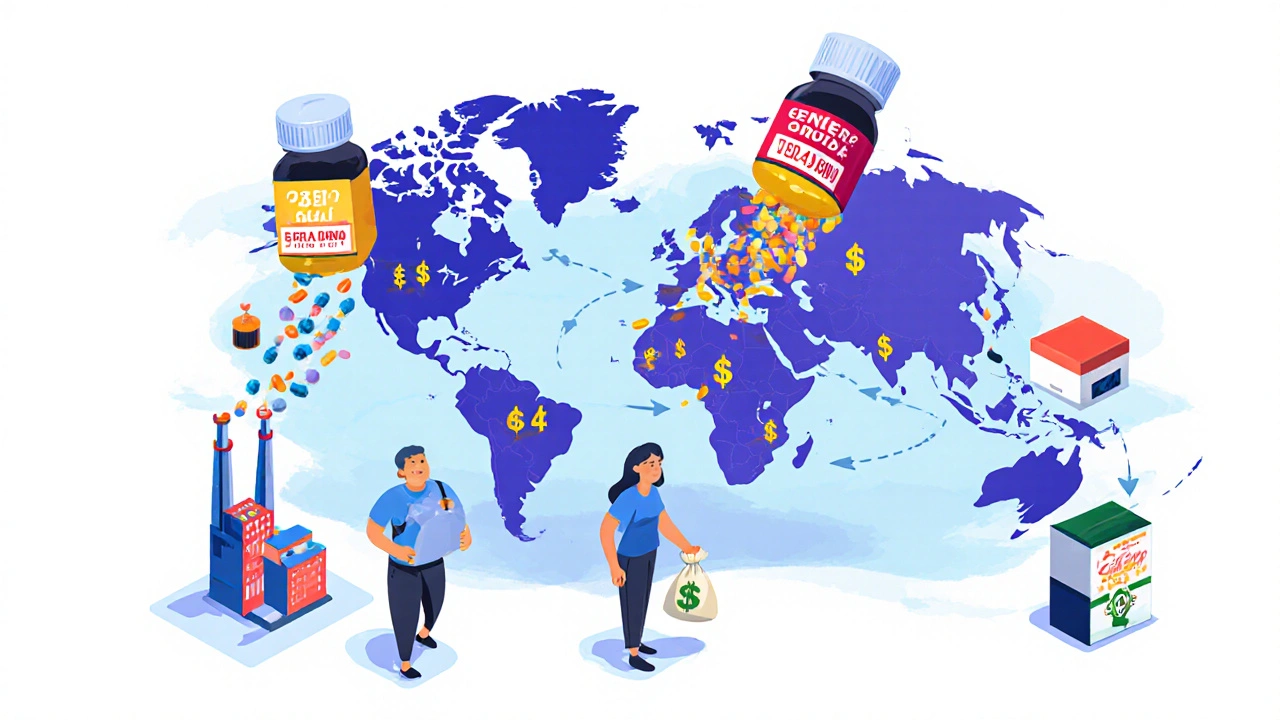
The global generic drug market is set to grow to over $700 billion by 2030, driven by patent expirations, aging populations, and cost pressures. Biosimilars and complex generics are leading the next wave of affordability in healthcare.