Pharmacy: Your Guide to Safe and Convenient Medication Options
Finding the right pharmacy can feel tricky, especially online where fake sites lurk everywhere. Whether you're ordering prescription meds or looking for affordable options, knowing what to watch out for helps you stay safe and get what you need without hassle.
How to Spot a Safe Online Pharmacy
First off, check if the online pharmacy requires a prescription from your doctor. That's a huge sign they play by the rules. Also, look for clear contact info and professional certification badges on their site. If prices seem way too good to be true or they push you to buy without proper checks, walk away. These warning signs mean trouble. Sites like Pharmacy2Home UK show how safe ordering should work—easy, secure, and delivered fast.
Best Alternatives to Popular Canadian Pharmacies
Sometimes you want more options than the usual pharmacies offer. Luckily, 2025 brings fresh alternatives like RxOutreach that provide affordable medications, especially for those without insurance. Others specialize in treating specific conditions or offer unique discounts. It's smart to explore these choices before deciding, so you find the best fit for your needs and budget. Checking recent reviews and how user-friendly the pharmacy sites are can help you pick wisely.
Remember, buying medication online saves time but demands you stay alert. Use promo codes carefully, verify the pharmacy’s reputation, and order only from trusted sources. This way, your health stays protected, and your wallet isn’t left empty. Canadian Pharma Hub keeps you informed with up-to-date info so you can shop confidently every time.
The FDA Orange Book lists approved generic drugs and tells pharmacists which ones can be safely swapped for brand-name drugs. It's the key to affordable medication in the U.S.
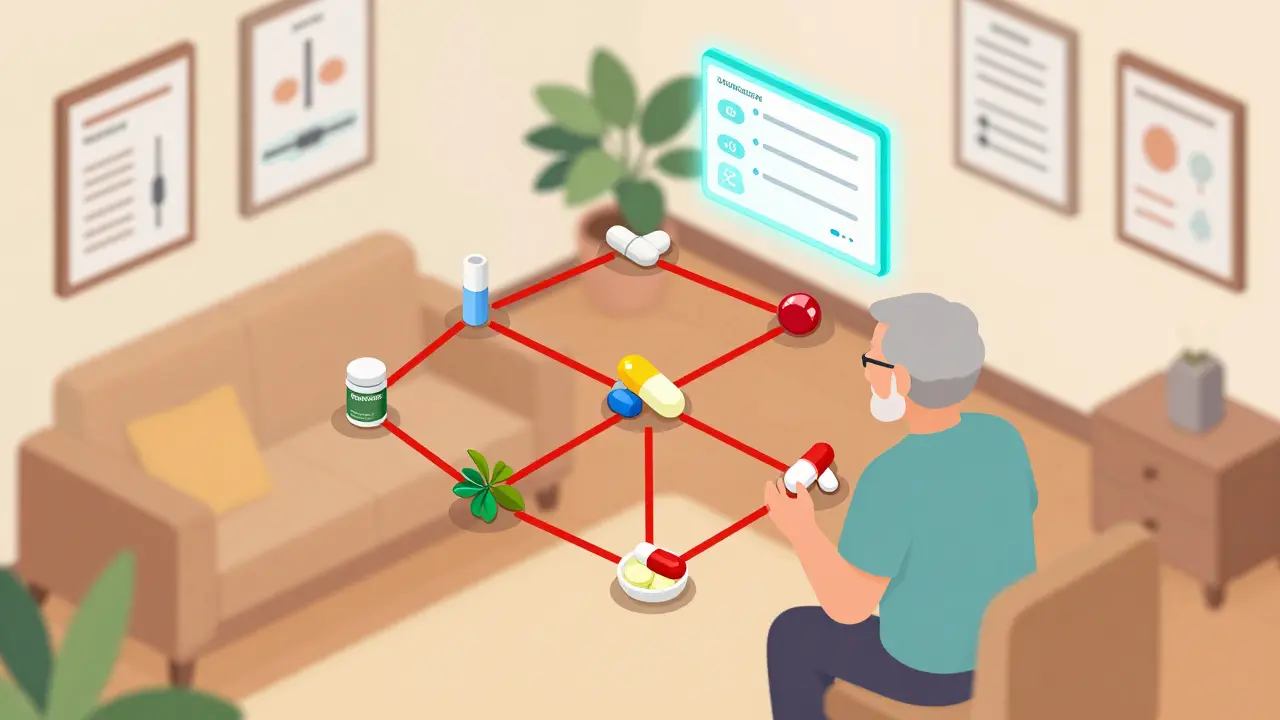
Digital tools now help prevent dangerous interactions between generic drugs, OTC meds, and supplements. Learn how Epocrates, Micromedex, and DDInter work - and why they’re changing patient safety.
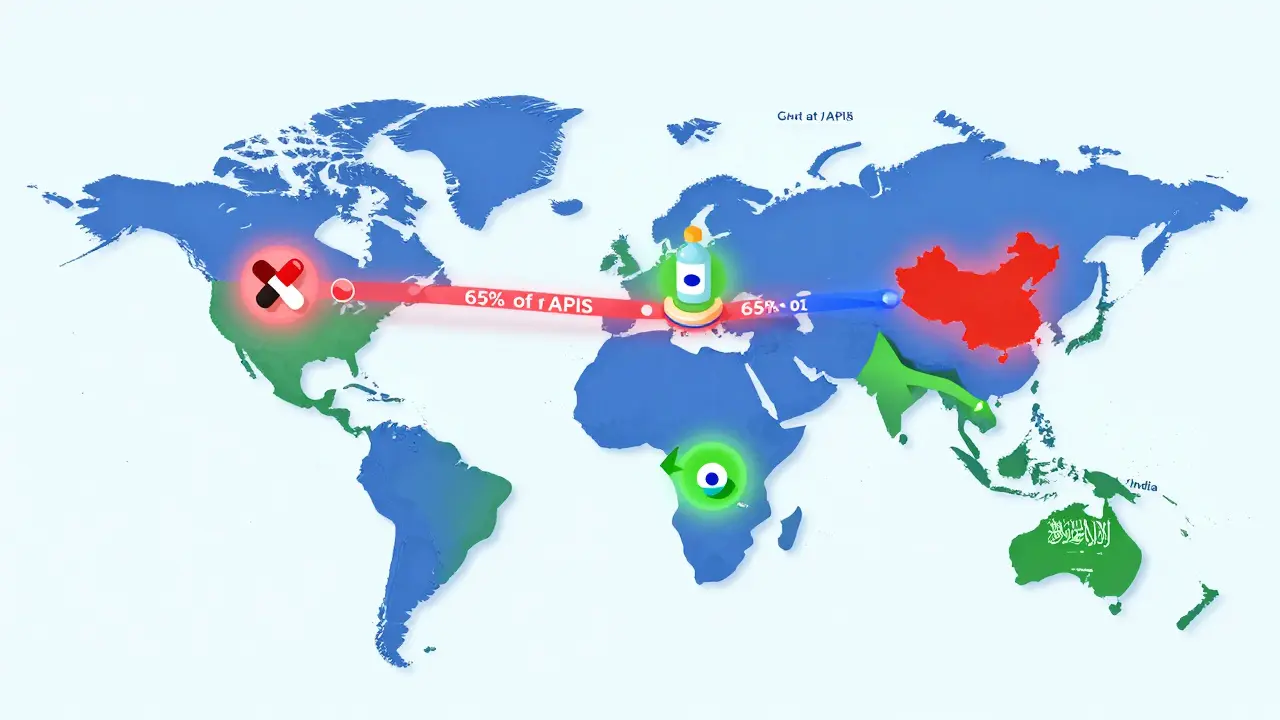
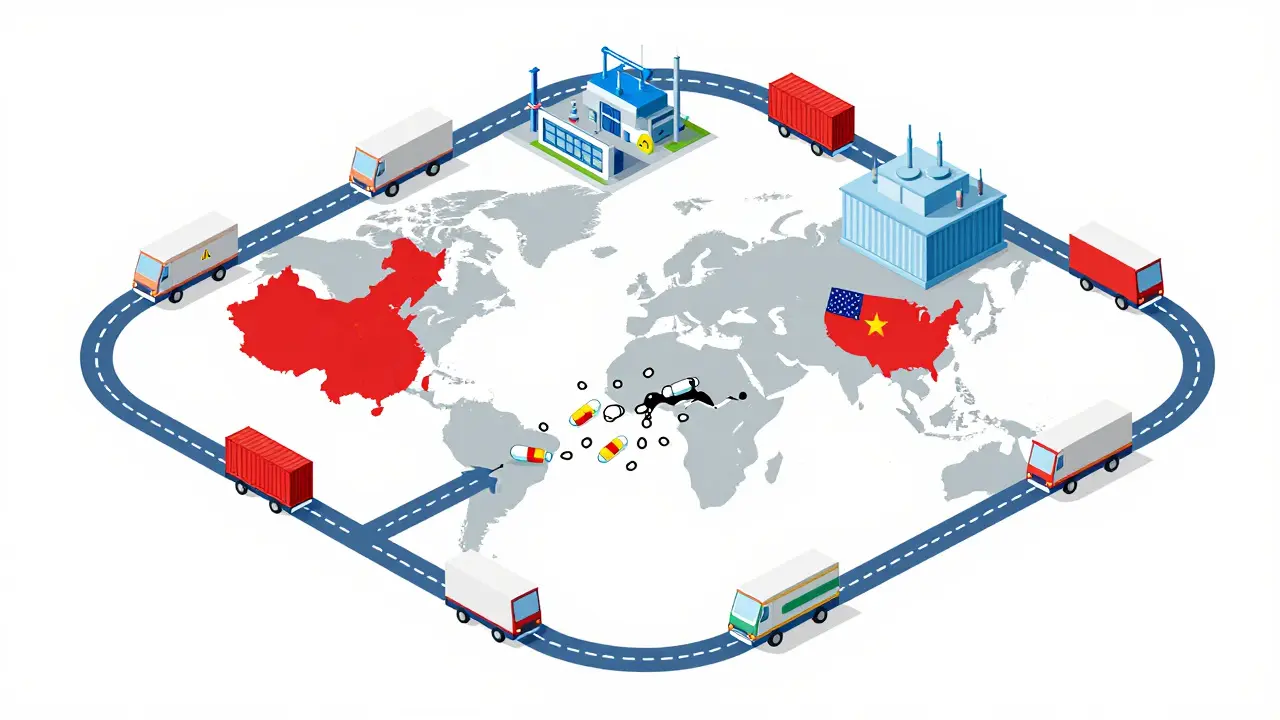
The global generic drugs market is evolving fast. With biosimilars rising, supply chains shifting, and emerging economies driving growth, affordability remains key - but quality and innovation are now just as important.
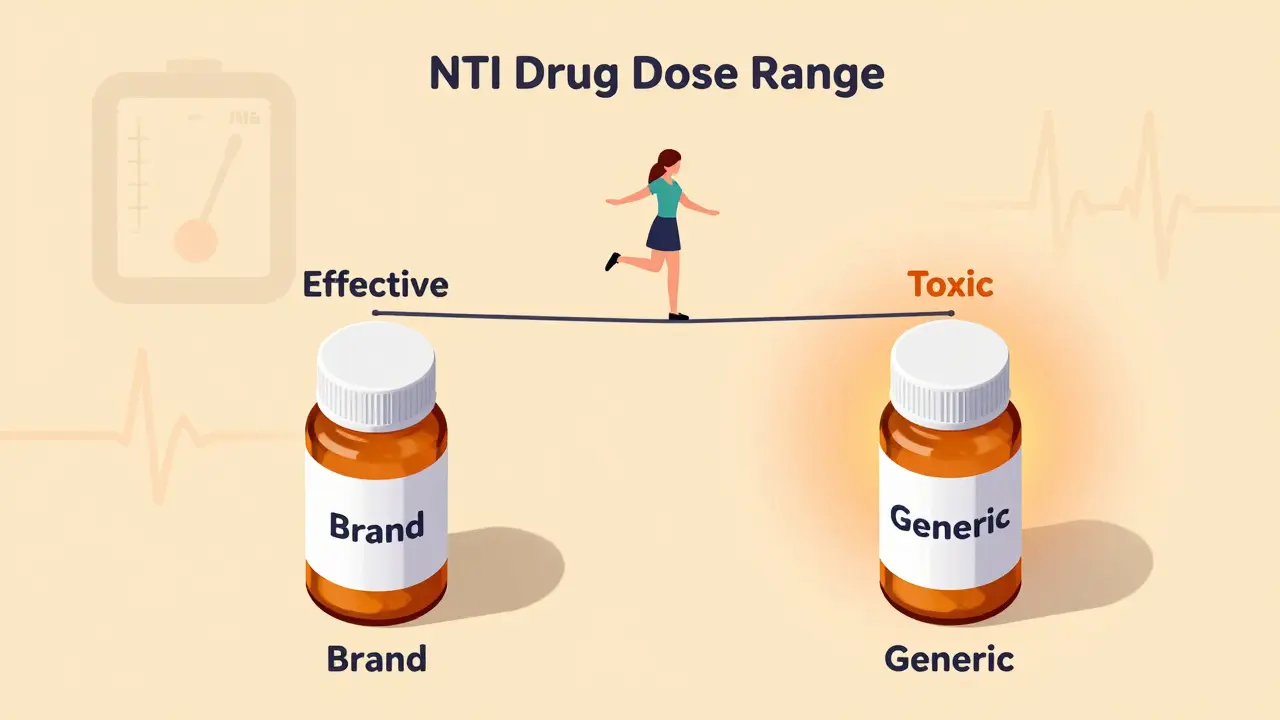
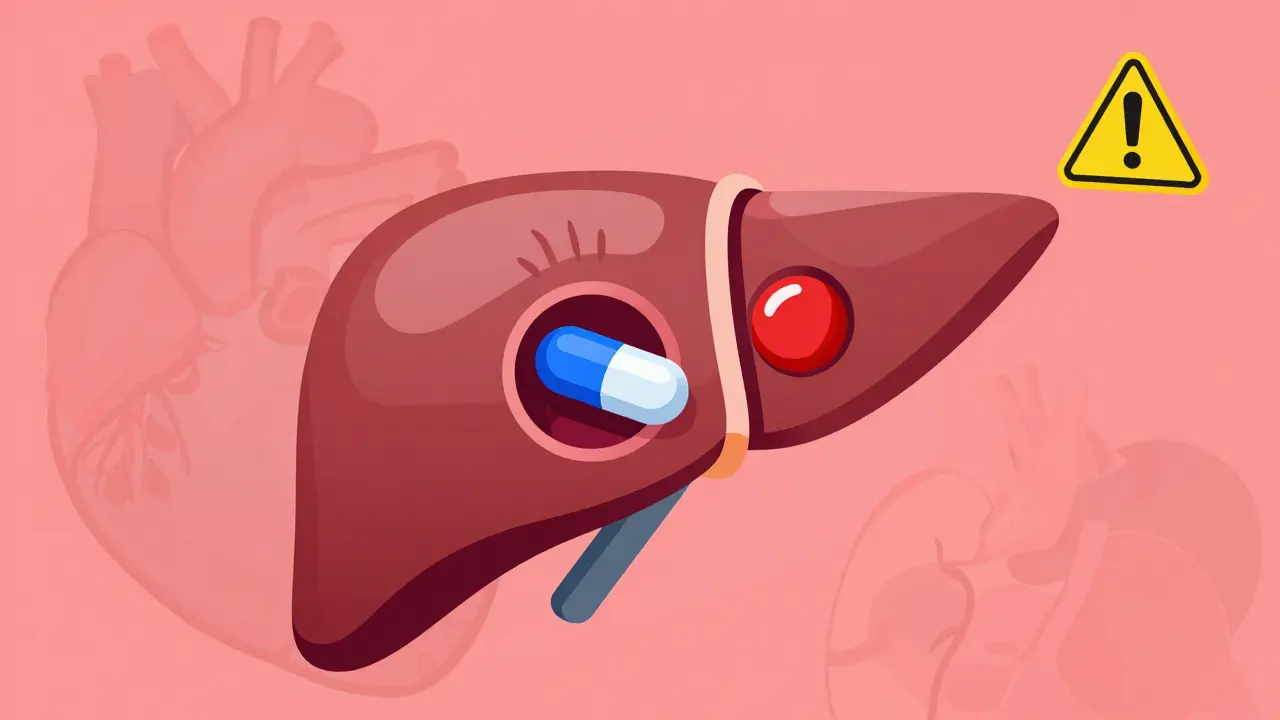
NTI drugs like warfarin and phenytoin have a tiny margin between effective and toxic doses. Generic substitutions can push patients out of this narrow range, risking serious harm - even death. Here’s why switching isn’t always safe.
Pharmacists can legally substitute generic drugs in all U.S. states, but therapeutic substitution rules vary widely. Learn how state laws shape what pharmacists can do, the documentation required, and why this matters for patient access and safety.
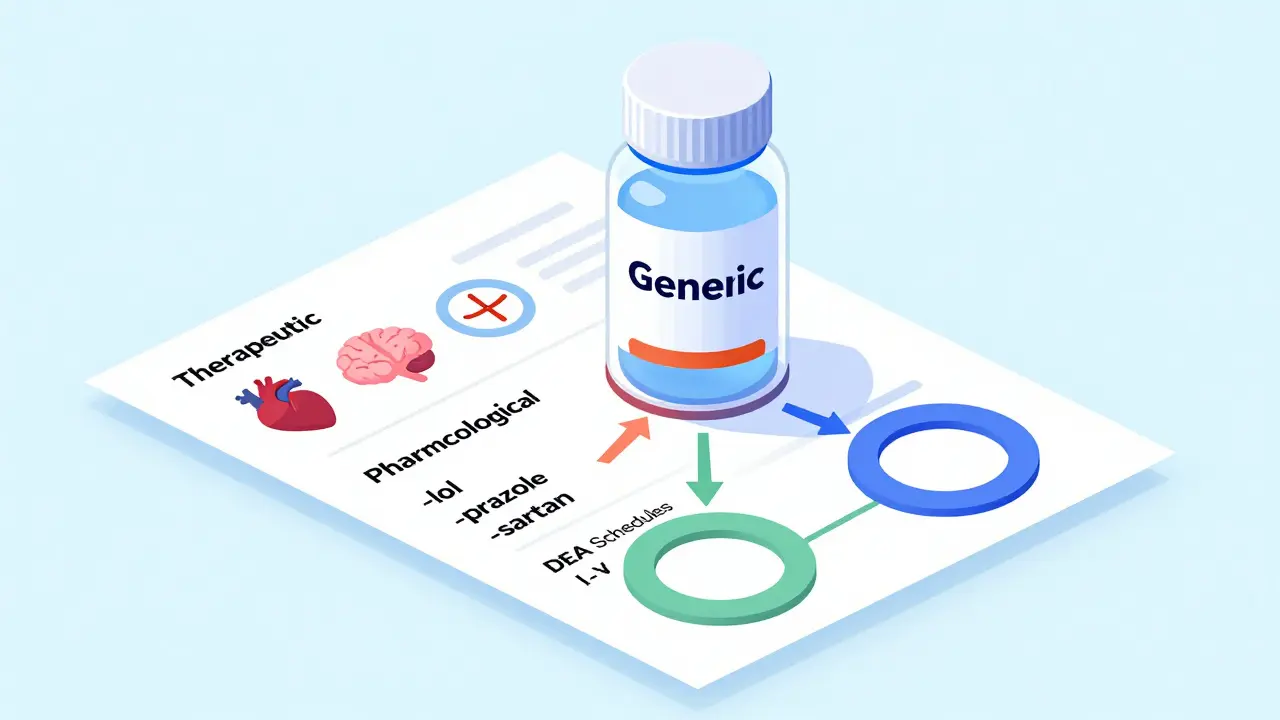
Generic drugs are classified by therapeutic use, mechanism of action, legal status, and insurance tiers. Learn how these systems work, why they matter, and how they affect your prescriptions.
Europe's tendering system for generic drugs prioritizes transparency, fairness, and value over lowest price. Learn how MEAT evaluation, framework agreements, and digital tools shape public pharmaceutical procurement across the EU.
Learn how to prevent drug shortages by building resilient pharmaceutical supply chains through buffer stocks, dual-sourcing, AI forecasting, and regional manufacturing. Real strategies for companies and policymakers.
Crossover trial designs are the gold standard for bioequivalence studies, using each participant as their own control to reduce variability and sample size. Learn how AB/BA and replicate designs work, when to use them, and why regulators require them.
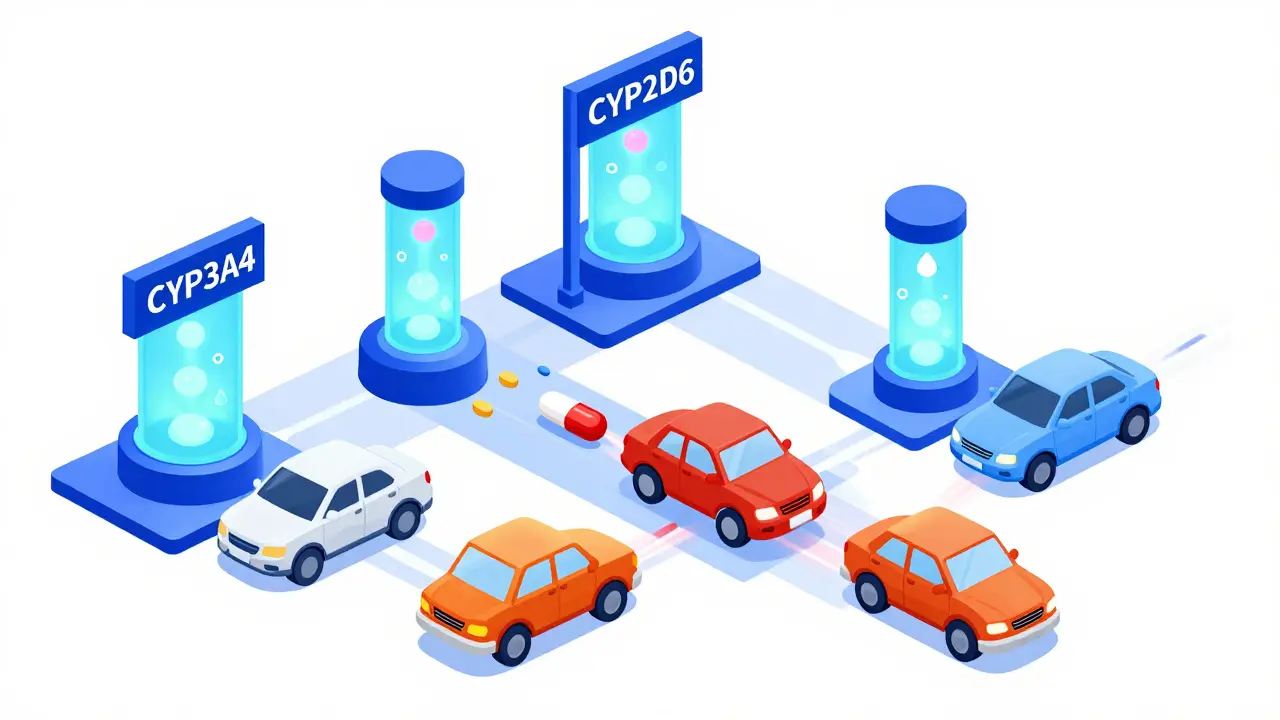
Omeprazole can reduce clopidogrel’s effectiveness by blocking the CYP2C19 enzyme needed for activation. Avoid omeprazole and esomeprazole-use pantoprazole instead. Genetic testing helps identify those at highest risk.
CYP450 enzymes process 90% of medications, but drugs often compete for them - leading to dangerous interactions. Learn how common meds like statins, antidepressants, and antibiotics clash in your liver - and what to do about it.
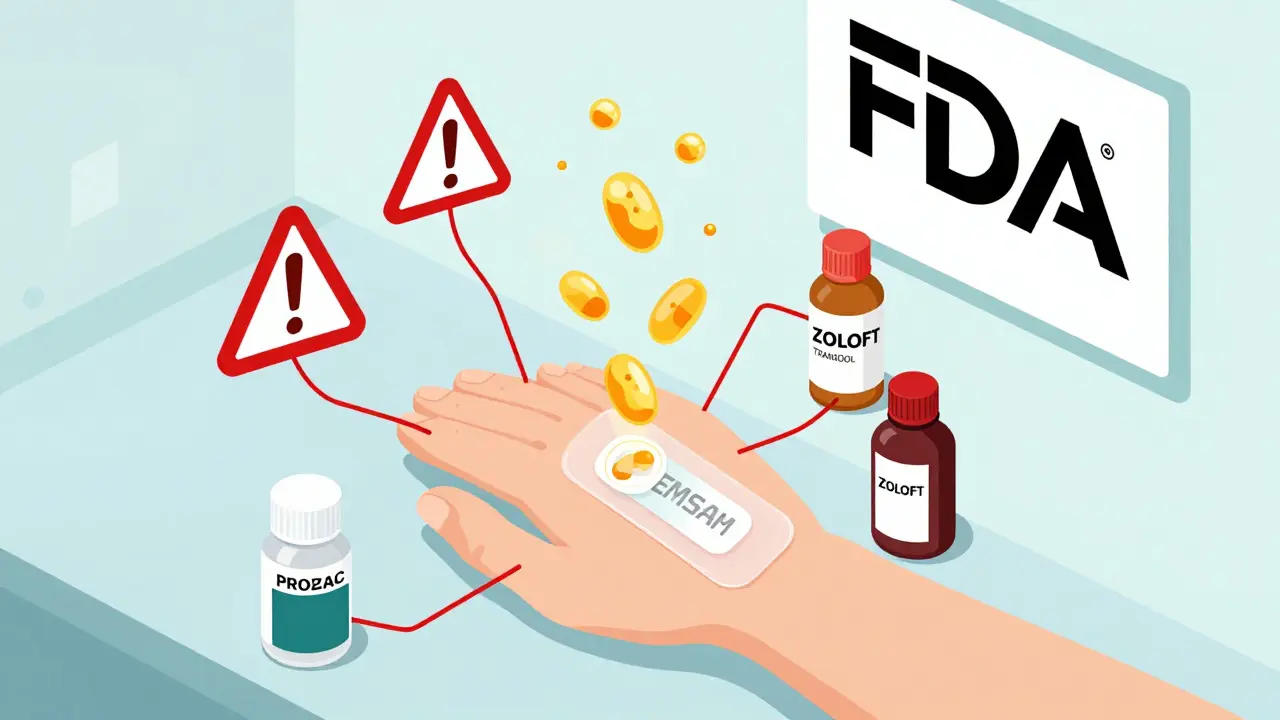
Selegiline transdermal (EMSAM) reduces dietary risks but still causes dangerous serotonin syndrome when mixed with common antidepressants, painkillers, and cold medicines. Learn the real washout periods, hidden triggers, and how to stay safe.